Introduction
The problem of phosphorous availability is becoming a great matter of concern and a major constraint for soil fertility in the majority of agricultural soils due to acidic nature of these soils. In these regions, phosphate ions are either adsorbed onto the surface of soil minerals or precipitated by free aluminum and iron, and this leads to widespread phosphorus deficiency (Frossard et al., 2000). Thus, the release of insoluble and fixed forms of phosphorus is an important aspect of increasing soil phosphorus availability. To overcome this problem, farmers used to apply a several-fold excess of phosphorus needed by the plants. This excess application of chemical phosphatic fertilizers causes environmental as well as economic problems.
A diverse group of soil microflora is reported to be involved in solubilizing insoluble phosphate complexes supplying plants with available phosphorus especially in soils with limited phosphorus (Tripura et al., 2005). Microorganisms that convert insoluble phosphates into soluble forms are termed to phosphate solubilizing microorganisms. Solubilization is achieved through acidification, chelation, ion exchange reactions, and production of low molecular weight organic acids such as gluconic, oxalic, and citric acids (Chaiharn and Lumyong, 2009). In addition to providing available phosphorus to plants, phosphate solubilizing microorganisms can enhance plant growth through several different mechanisms, such as symbiotic nitrogen fixation, ammonia production, production of plant hormones, and control of phytopathogenic microorganisms (Rangarajan et al., 2003). Therefore, the use of microorganisms with higher phosphate solubilizing abilities has proved to be an economically sound alternative to the more expensive superphosphates and thus possesses a greater agronomic utility (Khan et al., 2007).
It has been reported that plant growth promoting rhizobacteria (PGPR) including phosphate-solubilizing microorganisms (PSMs) are able to solubilize the unavailable forms of P in soil by acidification, chelation, and exchange reaction in the soil environment (Maliha et al., 2004; Ponmurugan and Gopi, 2006). However, soil is inhabited by several diverse groups of soil microorganisms and there is competition between different soil microorganisms owing to synergistic and antagonistic interactions (Sylvia et al., 2005). Metabolic activity, nutrient requirements of microorganisms, and environmental factors are involved in determining the dominance of species of organisms within the soil. PSMs also exhibit synergistic and antagonistic interactions with each other and, therefore, it is important to understand the way PSMs compete or cooperate with each other in soil before use them as bio-fertilizers.
PSMs have been widely used as inoculants to increase phosphorous uptake and crop yield and there are several previous reports regarding plant growth promotion and increase of phosphorous availability due to co-inoculation of PSMs under green house as well as field conditions (Reyes et al., 2002; Zaidi et al., 2003; Khalid et al., 2004; Hameeda et al., 2006; Chen et al., 2008). However, more adequate laboratory methods are needed for better understanding of the interactions of the inoculated microorganisms in the soil.
This study evaluates the effect of co-inoculation of phosphate solubilizing bacteria, Burkholderia anthina and Enterobacter aerogenes on solubilization of inorganic phosphate in the growth medium and their effect on growth and nutrient uptake of green gram plants grown under greenhouse conditions.
Materials and Methods
Isolation and identification of bacterial strains
The soils used for isolating phosphate solubilizing bacteria were collected from greenhouse soils of Chungchugnam-do province, Gongju-Gun area, in South Korea. Greenhouse soil was mixed with a sterile 0.85% NaCl solution and shaken for 30 minutes. Serial dilutions were used to inoculate NBRIP (National Botanical Research Institute Phosphorus) agar plates containing 10 g glucose, 5 g Ca3(PO4)2, 5 g MgCl2.6H2O, 0.25 g MgSO4.7H2O, 0.2 g KCl, 0.1 g (NH4)2SO4 in 1 L distilled water (Nautiyal, 1999). The plates were incubated for 5 days at 30℃. The colonies with clear halos were considered to be phosphate solubilizing colonies. Two predominant bacterial strains (PSB-15 and PSB-16) that exhibited large clear zones on the agar plates were selected as the efficient phosphate solubilizing organisms for further study.
The partial sequencing of 16S rRNA for the bacterial strains was done with the help of a DNA sequencing service, SOLGENT, Daejeon, South Korea, using universal primers, 27F (5’-AGAGTTTGATCCTGGCTCAG-3’) and 1492R (5’-GGTTACCTTGTTACGACTT -3’). The online program BLAST was used in identifying the related sequences with known taxonomic information available at the NCBI databank (http://www.ncbi.nlm.nih.gov/BLAST). A Phylogenetic tree was constructed using the CLUSTAL X program (Thompson et al., 1997), which involved sequence alignment by neighbor joining method (Saitou and Nei, 1987) and maximum parsimony using the MEGA4 program (Kumar et al., 2001). Grouping of sequences was based on confidence values obtained by bootstrap analysis of 1,000 replicates. Gaps were edited in the BioEdit program and evolutionary distances were calculated using the Kimura two parameter model. Reference sequences were retrieved from GenBank under the accession numbers indicated in the trees.
Inoculum preparation and inoculation
A single colony was transferred into 100 mL flasks containing 25 mL nutrient broth and grown aerobically in flasks on a rotating shaker (150 pm) for 48 h at 30℃. The bacterial suspension was then diluted in sterile distilled water to a final concentration of 108 CFU mL-1 and resulting suspensions were used to inoculate sterilized 500 mL Erlenmeyer flasks (n = 3) containing 200 mL National Botanical Research Institute Phosphorus (NBRIP) liquid medium as single bacterial inoculation. Flasks were incubated for 8 days with continuous shaking at 30℃. Sterilized uninoculated medium served as a control. A 10 mL sample was taken from each culture and control at 2, 5, and 8 days after inoculation and transferred into centrifugation tubes for centrifugation at 8,000 rpm for 10 min. The supernatant was used to determine the level of phosphorous release, pH, residual glucose content, and organic acid production.
Solubilization Index
A pin point inoculation of each bacterial strains preserved in sterilized 30% glycerol was placed on NBRIP agar plates (n = 3) under aseptic conditions and incubated at 30℃ for 7 days. Solubilization Index was measured daily using following formula (Edi-Premono et al., 1996).
Assay of phosphorous release and medium pH
Phosphorous release into the medium was assayed using the phospho-molybdate blue color method (Murphy and Riley, 1962). The pH of the culture medium was recorded with a pH meter equipped with a glass electrode.
Assay of residual glucose content
The residual glucose content of the culture medium was assayed using the DNS (3,5-dinitrosalicylic acid) method as described by Miller (1959).
Assay of organic acid production
To determine the organic acid composition of the different cultures, aliquots from supernatants were analyzed using high-performance liquid chromatography (Agilent, 1260 infinity, USA). The column used for analysis was Inertsil ODS 3V, and a UV detector was set to 210 nm at 40℃. Mobile phase consisted of 0.008 M H2SO4 run at a flow rate of 0.2 mL min-1. HPLC profiles of the culture filtrates were analyzed by comparison with the elution profiles of pure organic acids (gluconic acid, oxalic acid, and citric acid) injected separately. Peaks were identified by retention times against a set of standards from known three organic acids.
Inoculum preparation for pot experiment
A single colony was transferred into 500 mL flasks containing nutrient broth and grown aerobically on a rotating shaker (150 rpm) for 48 h at 30℃. The bacterial suspension was then diluted in sterile distilled water to a final concentration of 108 CFU mL-1 and resulting suspensions were used to treat green gram seeds. For dual inoculation, equal volumes (108 CFU mL-1 of each inoculant) of the two cultures were mixed, and then used to treat green gram seeds (the same as for single inoculation).
Plant growth promotion bioassay in Pot experiment
The experiment was carried out in a greenhouse located at the Chungnam National University, South Korea. The potting soil was classified as sandy loam and had the following characteristics: pH 6.55, NH4+-N 300 mg kg-1, NO3--N 300 mg kg-1, P2O5 255 mg kg-1, and CEC 10 Cmol+ L-1. The pots were filled with this soil (25 cm diameter, 35 cm height), and basal doses of nitrogen (50 mg kg-1 soil) and potassium (120 mg kg-1 soil) were applied in the form of urea and potassium chlorite, respectively. Tricalcium phosphate (TCP) was supplied as phosphate fertilizer in the dose of 160 mg kg-1 soil based on nutrient requirements of green gram plants. The pots were arranged in a completely randomized block design with three replications per treatments. The experimental plot was based on eight treatments as follows: (1) Soil without TCP, PSB-15, and PSB-16; (2) Soil + TCP; (3) Soil + PSB-15; (4) Soil + PSB-15 + TCP; (5) Soil + PSB-16; (6) Soil + PSB-16 + TCP; (7) Soil + PSB-15 + PSB-16; and (8) Soil + PSB-15 + PSB-16 +TCP.
Green gram (Vigna radiate var. paiyur 1) seeds were surface sterilized by immersing in 0.1% sodium hypochlorite solution for 10 minutes and then washed thrice with distilled water. The top 15 mm of soil from earthen pots was removed and six seeds were placed in the pot at equal distances. A 1 mL of each of the initially prepared inoculants was uniformly applied as single- and co-inoculation on the seeds, and then seeds were covered with a 15 mm thick soil layer. Control plants received 1 mL of diluted nutrient broth with no bacteria. Pots were watered daily to maintain the water holding capacity of the soil during the study period. After one week of germination, plants were thinned out to 3 plants per plot. Growth promotion effects of bacterial treatments were assessed by measuring shoot and root length, shoot and root weight, and P uptake of green gram plants. The root and shoot portions of plants were separated and air dried before being placed in an oven at 70℃. The shoot and root dry weights were measured separately and the average weight of three plants were expressed as g plant-1. Plant samples were finely ground after drying and used to determine phosphorous content of plants, following the Vandomolybdate phosphoric yellow color method as described by Jackson (1973).
Statistical analysis
Values were given as means ± SD for triplicate samples. The data were subjected to analysis of variance (ANOVA) using SAS package (SAS, 1999). The Duncan’s Multiple Range Test (DMRT) was applied to test the significance of treatment means at p ≤ 0.05.
Results
Isolation and identification of bacterial strains
The two selected bacterial strains (PSB-15 and PSB-16) had marked phosphate solubilizing abilities as visualized by the clear zone development around the colonies after 3 days of incubation. Based on 16S rRNA sequence analysis, the strains were identified as Burkholderia anthina and Enterobacter aerogenes. Comparison of the 16S rRNA sequence among available strains of Burkholderia and Enterobacter species showed high homology (> 99%) to Burkholderia anthina AJ420880 and Enterobacter aerogenes KCTC2190 (data not shown). Two strains (PSB-15 and PSB-16) were named as Burkholderia anthina PSB-15 and Enterobacter aerogenes PSB-16, respectively, based on the relationships between 16S r RNA sequence of strains and other Burkholderia and Enterobacter species.
Effect of carbon and nitrogen sources
Nutrition condition of the culture medium affects microbial growth as well as phosphate solubilization (Jain et al., 2012). Inorganic phosphate solubilizing capacity of PSB-15 and PSB-16 strains was assessed in the presence of eight carbon sources and five nitrogen sources by replacing glucose and (NH4)2SO4, respectively, in the NBRIP medium (Table 1). Burkholderia anthina PSB-15 and Enterobacter aerogenes PSB-16 showed diverse levels of phosphate solubilizing activity in the presence of various carbon and nitrogen sources. The test of various carbon sources on phosphate solubilization revealed that glucose was the preferred source for two strains followed by galactose (PSB-15: 90.3%, PSB-16: 92.6% of the control). Xylose was found to be a poor source of carbon for two strains in solubilization of phosphate. Corresponding to this result, it has been reported that glucose is the best carbon source for phosphate solubilization in Burkholderia anthina (Walpola and Yoon, 2013) and Pantoea species (Walpola et al., 2013).
Various ammonium and nitrate sources were added separately to the medium to assess their effects on phosphate solubilization and results are shown in Table 1. According to the results, it is clear that ammonium and nitrate sources are equally effective for phosphate solubilization by PSB-15 and PSB-16. In agreement with this, Nautiyal et al. (2000) also observed that all ammonium and nitrate sources were utilized for phosphate solubilization by NBRI0603, NBRI12601, NBRI13246, and NBRI14003 strains. However, there are some earlier reports contrary to these findings showing differences in phosphate solubilization as well as different mechanisms involved in acidity generation when ammonium and nitrate were used (Halder et al., 1991; Kpomblekou and Tabatabai, 1994). They reported that the rate of phosphate solubilization accelerates due to inorganic acids production by a proton exchange mechanism in the presence of ammonium ion (Ahuja et al., 2007). However, production of organic acids was greatly affected by the carbon source, not the nitrogen source. Therefore, organic acid production is not the sole factor responsible for phosphate solubilization.
Phosphate solubilization under in vitro conditions
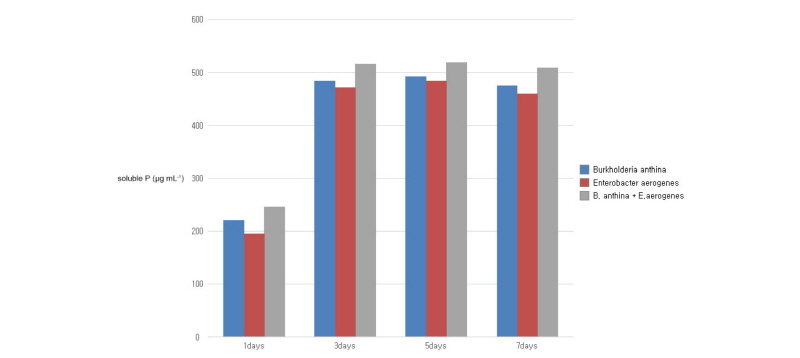
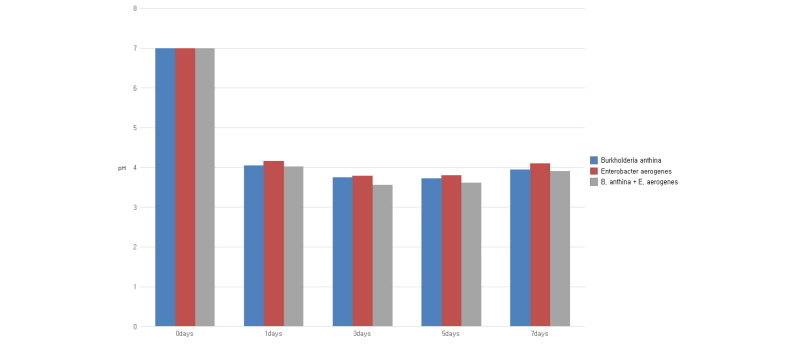
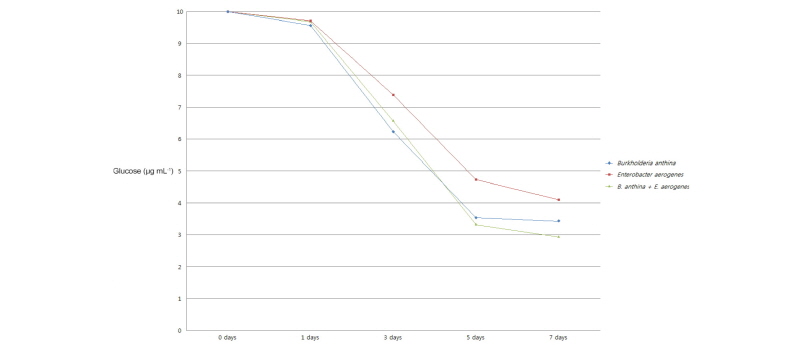
The periodic changes in soluble phosphorus content released from the pH buffer of the culture, Ca3(PO4), and residual glucose content in NBRIP medium by single and co-inoculation of B. anthina PSB-15 and E. aerogenes PSB-16 during 8 days of incubation are presented in Fig. 1, 2, and 3, respectively. Significant (p ≤ 0.05) increments in soluble phosphorous content were observed with inoculation of PSBs. Co-inoculation of two strains was shown to release the highest content of soluble phosphorus (519 µg mL-1) into the medium, followed by single inoculation of B. anthina and E. aerogenes with 492 and 483 µg mL-1 of soluble phosphorus, respectively (Fig. 1). Although there was no significant difference between single inoculation and co-inoculation of bacterial strains in terms of phosphorous release, the results clearly depict that co-inoculation enhanced phosphate solubilization in the liquid culture medium. This is in agreement with Bras and Nahas (2012) who observed a similar trend of phosphate solubilization with Burkholderia cepacea and Aspergillus niger co-inoculated medium. The average phosphorous release of their experiment was recorded as 570 µg mL-1 for bacteria, 740 µg mL-1 for fungi, and 760 µg mL-1 for the co-culture at the end of the incubation period. Our results also showed that the content of soluble phosphorus released by all inoculants in the culture medium increased significantly during the first 5 days of the incubation and remained high for a further few days. The single and co-inoculation of B. anthina and E. aerogenes caused a pH reduction in the culture medium (Fig. 2). In bacteria inoculated medium, the pH was reduced from 7.0 to 3.74 after 3 days of incubation. In co-inoculated medium, it was reduced to 3.51 after 3 day’s incubation. Both single and co-inoculation showed negative correlation (p ≤ 0.05) between soluble phosphorus content and pH in the culture medium. A similar strong negative correlation was observed between residual glucose content and phosphate solubilization. The glucose contents in B. anthina and co-inoculation media were consumed to 61.9% and 64.7% after 5 days of incubation, respectively (Fig. 3).
|
|
|
Fig. 1. Effect of single and co-inoculation of Burkholderia anthina PSB-15 and Enterobacter aerogenes PSB-16 on phosphate solubilization. Values given here are the means (n = 3) ± standard deviation. |
|
|
|
Fig. 2. Effect of single and co-inoculation of Burkholderia anthina PSB-15 and Enterobacter aerogenes PSB-16 on pH. Values given here are the means (n = 3) ± standard deviation. |
|
|
|
Fig. 3. Effect of single and co-inoculation of Burkholderia anthina PSB-15 and Enterobacter aerogenes PSB-16 on residual glucose content. Values given here are the means (n = 3) ± standard deviation. |
Organic acid produced by PSBs
The organic acid production by single- and co-inoculation of B. anthina and E. aerogenes was evaluated with HPLC analysis. As shown in Table 2, gluconic acid was the major organic acid produced by both single and co-inoculated culture media, followed by oxalic acid and citric acid. However, two bacterial strains were not capable of citric acids unlike other strains. Similarly, to phosphate solubilization, no significant difference was observed in organic acid production between single inoculation and co-inoculation of bacterial strain. In agreement with the present study, gluconic acid was the major organic acid produced by Burkholderia cepacia DA23 (Song et al., 2008), Burkholderia cepacia CC-A174 (Lin et al., 2006), and Aspergillus flavus and Aspergillus niger (Maliha et al., 2004). Singh and Amberger (1991) reported the production of significant levels of glycolic acid, oxaloacetic acid, succinic acid, fumaric acid, malic acid, tartaric acid, and citric acid by Aspergillus niger during straw composting with rock phosphate. Organic acid production increased with the incubation period, reaching to the maximum at day 5. Similarly, a decrease in pH and glucose content and an increase in soluble phosphorous content were observed during the same period. Therefore, this inverse relationship between phosphate solubilization and organic acid production with pH suggested that production of organic acids played a significant role in the acidification of the culture medium.
Growth and phosphorous uptake in green gram plants
Increased shoot length, root length, and shoot and root dry weight of green gram plants were recorded from the seedlings raised with the PSB inoculated seeds (Table 3). The best growth performances (33.08 cm, 34.05 cm, 3.83 g and 3.35 g plant-1, respectively, for shoot length, root length, shoot dry weight, and root dry weight) were recorded from the plants co-inoculated with B. anthina PSB-15 and E. aerogenes PSB-16 amended with TCP. Although addition of TCP resulted in better growth performances, no significant (p ≤ 0.05) differences in shoot length, root length, shoot dry weight, and root dry weight were observed between treatments with and without TCP (Table 3).
As shown in Table 4, P uptake of green gram plants showed a similar trend to the growth parameters. Increases in P uptake of shoot, root, and total P uptake was observed in plants inoculated with B. anthina PSB-15, E. aerogenes PSB-16 or both strains. Moreover, the addition of TCP to PSB inoculated seeds significantly (p ≤ 0.05) increased shoot and root phosphorous uptake. Co-inoculation of PSB strains with TCP further improved phosphorous uptake compared to single inoculation with any of the PSB strains with TCP incorporation. The maximum P uptake (166.32 and 70.27 mg plant-1, respectively, for shoot and root) was recorded from co-inoculated plants with TCP. There was no significant difference (p ≤ 0.05) between un-inoculated seeds treated with and without TCP. However, pH reduction in soil was found to be much lower than that in the culture medium (Fig. 2), which could be due to the buffering nature of the soil used for the experiment. According to the plant growth promotion assay, both single- and co-inoculation of PSB strains had significantly different effects on shoot and root growth when compared with un-inoculated seeds. Although no significant difference was observed in phosphate solubilization between single and co-culture medium, a significant difference in shoot and root length was observed between single- and co-inoculation during the plant growth promotion assay. This may be due to enhanced phosphorus nutrition and other plant growth promoting activities due to synergistic action with the co-inoculated medium. However, considering a short assessment period of the present study, we recommend engaging in further work under field conditions in order to test the suitability of the strains to be used as bio-inoculants.
Changes in pH, available phosphorous and PSB population
Table 5 presents the effect of single and co-inoculation of PSB strains on pH, available phosphorous content, and total PSB population. A more significant decrease (p ≤ 0.05) in soil pH was recorded from PSB inoculated soils than un-inoculated soils. However, no significant (p ≤ 0.05) difference in soil pH was observed between single- and co-inoculated soils. Furthermore, available phosphorous content of rhizosphere soil inoculated with either a single PSB or both strains was found to be significantly (p ≤ 0.05) higher than those in un-inoculated soil. This was further enhanced by the addition of TCP. The highest available phosphorous content (182.39 mg kg-1 soil) in co-inoculated soil with TCP was 1.8 times higher than that in un-inoculated soil. A remarkable increase in PSB population was observed in the PSB inoculated rhizosphere soil when compared with un-inoculated soil. The PSB population (6.45 × 106 CFU g-1 soil) in PSB co-inoculated soil with TCP was approximately 2 times higher than that in un-inoculated soil. In conclusion, significant (p ≤ 0.05) increments in soluble phosphorous content, titratable acid production, and microbial growth were observed with PSB inoculation. Significant reduction in the pH of the PSB inoculated medium was also observed compared to the control where it remained constant. Co-inoculation of two PSB strains showed higher phosphate solubilization than single inoculation. A strong negative correlation between phosphate solubilization and pH, as well as a strong positive correlation between phosphate solubilization and microbial growth could also be observed.
Discussion
Phosphate solubilizing bacteria (PSB) were reported to solubilize insoluble phosphate complexes in order to reduce the pH of their surroundings either by releasing organic acids or protons (Hariprasad and Niranjana, 2009). Organic acids such as gluconic acid, oxalic acid, and citric acid, secreted by PSB, can directly solubilize mineral phosphate as a result of anion exchange or indirectly chelate both Fe and Al ions associated with phosphate. This leads to an increase of phosphorus to plants, especially in soils with limited phosphorus (Tripura et al., 2005). Studies in liquid cultures revealed that phosphate solubilizing microorganisms increased the content of available phosphorus by solubilizing suspended tricalcium phosphate (TCP) due to the release of organic acids into the surrounding medium (Gaur, 1990). Previous reports also described some Burkholderia and Pantoea strains as efficient phosphate solubilizers (Khalimi et al., 2012). In the present study, two efficient PSB strains (Burkholderia anthina PSB-15 and Enterobacter aerogenes PSB-16), which had a marked insoluble phosphate solubilizing ability, were isolated and reaffirmed to be involved in the production of organic acids. The negative correlation between the pH and soluble phosphorous content of the medium, as well as the positive correlation between soluble phosphorous content and titratable acid production, suggested that acidification of the medium could facilitate phosphate solubilization. Comparatively, co-inoculation showed higher phosphate solubilizing enhancement than single inoculation, suggesting that both strains acted synergistically in phosphate solubilization. Yu et al. (2011) also found similar findings after inoculation of Pseudomonas chlororaphis and Bacillus megaterium.
Increased growth and phosphorous uptake of several crop plants due to inoculation of PSB have also been reported in a number of studies conducted both under growth chamber and green house conditions (Vikram and Hamzehzarghani, 2008; Hariprasad and Niranjana, 2009; Yu et al., 2011). The increases in shoot length, root length, shoot dry weight, and root dry weight of green gram plants inoculated with PSB strains could be attributed to a greater absorption of nutrients, especially phosphorous. Co-inoculation treatments resulted in higher growth performances and phosphorous uptake than those from single inoculation, suggesting that both strains acted synergistically in promoting green gram plant growth. However, phosphate solubilization is not the only way PSB promote plant growth, because they facilitate the growth of plants by stimulating the efficiency of producing plant hormones such as auxins, cytokinins, gibberellins as well as some volatile compounds (Podile and Kishor, 2006). Therefore, enhanced plant growth after inoculation of PSB strains may be attributed to the ability of strains to make phosphorous available and to simultaneously produce plant growth promoting substances (Khalid et al., 2004; Linu et al., 2009; Ali et al., 2010). Both strains used in this study exhibited the capacity to produce indoleacetic acid (data not shown) and, therefore, might have contributed to enhanced shoot and root lengths. Similar increases in growth and phosphorous uptake of green gram plants due to inoculation of PSB strains was observed by Ghanem and Abbas (2009). Ghanem and Abbas (2009) observed an increase in plant height, number of branches, number of pods, grain weight, and eventually, higher seed and straw yields in green gram plants after inoculation of Bacillus megaterium in salt affected soils. Increased growth and phosphorous uptake have been reported in wheat from Azotobacter chroococum (Kumar et al., 2001), in peanut from Pseudomonas fluorescens (Dey et al., 2004), in walnut from Bacillus cereus and Pseudomonas species (Yu et al., 2011), and in tomato from Paenibacillus polymyxa and Bacillus megaterium (EI-Yazeid and Abou-Aly, 2011). According to Fernandez et al. (2007), the shoot length of soybean plants was increased after inoculation with Burkholderia sp. PER2F by 40% and 60% compared to un-inoculated soil/seed and un-inoculated soil/seed treated with soluble P, respectively.
Present results of maximum plant growth and phosphorous uptake (Table 4), recorded when co-inoculating two PSB strains with TCP, are in line with the findings of Qureshi et al. (2011). They observed similar results when co-inoculating phosphate solubilizing and nodule forming bacteria Rhizobium phaseoli and Bacillus megaterium onto green gram plants. In conclusion, the inoculation of two PSB-15 and PSB-16 strains into soil contributed to the increase of total available phosphorous in soil through the production of organic acids and promoted the uptake of phosphorous by green gram plants.
Conclusion
This study has provided evidence that two PSB strains (Burkholderia anthina PSB-15 and Enterobacter aerogenes PSB-16) have significant effects on both phosphorus solubilization and plant growth enhancement by lowering pH along with the production of organic acids. Co-inoculation of two PSB strains could act synergistically and was responsible for the increase in several growth parameters in comparison with single inoculation. However, further studies should be carried out under field conditions, in confirmation of the present results, to demonstrate these strains’ high potential for use as soil inoculants to enhance soil fertility and plant growth.