Introduction
Soybean meal has been widely used as a protein source for monogastric animal in the world (FAO, 2004). Soybean contains not only abundant amounts of proteins but also plenty of
carbohydrates that are mainly composed of non-starch polysaccharides (NSP) and oligosaccharides (Choct, 1997). Soybeans consist of approximately 20% NSP, in which soluble and insoluble represent at about 3 and 17%, respectively (Choct, 1997).
Weaning is a stressful event for nursery pigs and is accompanied by tremendous changes in gastrointestinal physiology, microbiology, and immunology (Hampson, 1986; Pluske et al., 1997; Brooks et al., 2001). Moreover, the digestive system of weaned pig is not fully developed, and the secretion of digestive enzymes is limited to the digestion of dietary nutrients (Song et al., 2015; Park et al., 2016). Thus, weaned pigs have difficulty digesting diets based on corn and soybean meal with high amount of NSP by their endogenous enzymes. It is well known that unhydrolyzed NSP has negative effects on nutrient absorption (Song et al., 2015; Park et al., 2016). Supplementation of exogenous enzymes can be a solution to this problem. Therefore, the objective of this study was to evaluate the effects of supplementation with dietary enzyme cocktail containing carbohydrases and proteases on growth performance, intestinal morphology, and nutrient digestibility of weaned pigs.
Materials and Methods
The Chungnam National University Institutional Animal Care and Use approved all experimental protocols used in this study (approval code: CNU-00611).
Experimental design, animals, and diets
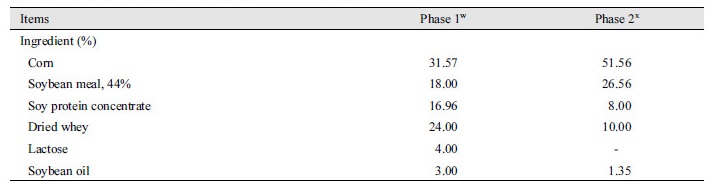
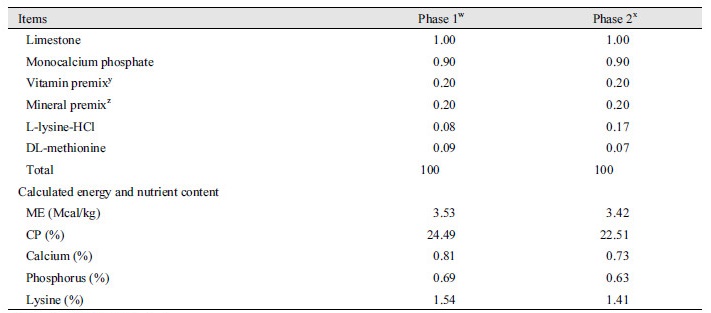
A total of 36 weaned pigs [Duroc × (Landrace × Yorkshire); 5.92 ± 0.48 kg of average body weight (BW); 28 d old] were used in this experiment. Pigs were moved to nursery pens equipped with a feeder and waterer in an environmentally controlled room and randomly assigned to 2 dietary treatments (3 pigs/pen, 6 replicated pens/ treatment) in a randomized complete block design. The dietary treatments were a normal corn and soybean meal based diet (CON) and CON with 0.05% enzyme cocktail [Cocktail; mixture of xylanase (4,000,000 U/kg), α-amylase (1,000,000 U/kg), protease (500,000 U/kg), β-glucanase (150,000 U/kg), and pectinase (25,000 U/kg)], which were commercially purchased. Pigs were fed for 6 wk using a 2-phase feeding program with declining diet complexity and each phase was lasted 3 wk. The diets did not include spray-dried plasma, antibiotics, or zinc oxide to avoid their antibacterial or physiological effects (Table 1). Pigs were allowed free access to diets and water at all times. For measurements of intestinal morphology, apparent total digestibility, and apparent ileal digestibility, 12 pigs were randomly selected (2 pigs from each pen).
Sample collection and analyses
Growth performance
The pigs were individually weighed at the start, on d 21, and 42 of the experiment and the average body weight gain was calculated. Amounts of feed supplied per pen were recorded for each phase and the rest of feed was weighed at the end of each phase.
Intestinal morphology
After the 6 wk experimental period, the 12 pigs selected as previously mentioned were euthanized using CO2 after injection of zoletil. Tissue samples from the small intestine were directly collected. Segments of ileum, about 5 cm in length, were taken and flushed with distilled water to take out any excess blood and digesta. Then, the tissues were immersed in a 6% formaldehyde phosphate buffer and kept at 4℃. The fixed intestinal tissues were embedded in paraffin, sectioned at a 5 µm thickness, and stained with hematoxylin and eosin. The slides were imaged using a digital slide scanner (NanoZoomer Digital Pathology System; Hamamatsu Co., Bridgewater, NJ). All measurements were conducted in the associated slide-viewing software (NDP. view; Hamamatsu Co.). The intestinal morphological measurements included villus height, crypt depth, villus area, and goblet cell number.
Apparent total tract digestibility
For the measurement of apparent total tract digestibility, chrome oxide, a indigestible marker, was added at 0.25% of the respective dietary treatments. The 12 pigs selected as previously mentioned were fed each dietary treatment with 0.25% chromic oxide between d 36 and 42 after weaning. Fecal samples were collected for 3 days post the 4-d adjustment period. Fecal samples were immediately stored at -20℃. Fecal samples were dried in a forced-air drying oven at 60℃ and ground through a cyclone mill (Foss Tecator Sycltec 1093, HillerØd, Denmark) before analysis. Fecal samples were analyzed for dry matter (method 930.15; AOAC, 2000), nitrogen (method 988.05; AOAC, 2000), and gross energy using a bomb calorimeter (Parr 1281 Bomb Calorimeter, Parr Instrument Co., Moline, IL, USA).
Apparent ileal digestibility
After the 6 wk experimental period, the 12 pigs selected as previously mentioned were euthanized using CO2 after injection of zoletil. Tissue samples from the small intestine were directly collected. Digesta samples were immediately stored at -20℃. Digesta samples were dried in a forced-air drying oven at 60℃ and ground through a cyclone mill (Foss Tecator Sycltec 1093, HillerØd, Denmark) before analysis. Digesta samples were analyzed for dry matter (method 930.15; AOAC, 2000), nitrogen (method 988.05; AOAC, 2000), and gross energy using a bomb calorimeter (Parr 1281 Bomb Calorimeter, Parr Instrument Co., Moline, IL, USA).
Statistical analysis
Data were analyzed using the GLM procedure of SAS (SAS Institute Inc., Cary, NC, USA) in a randomized complete block design. The pen was used as the experimental unit for the growth performance, intestinal morphology, and nutrient digestibility. The variability of the data was shown as the standard error and a probability level of p < 0.05 was considered to be statistically significant.
Results and Discussion
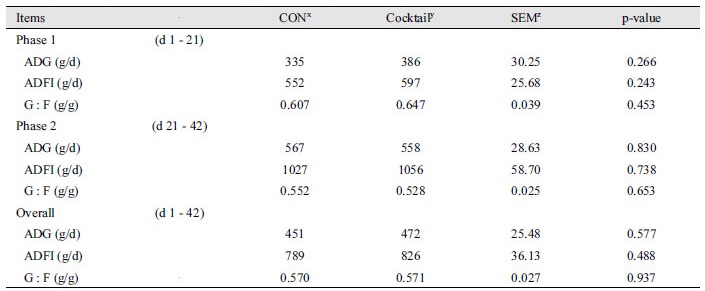
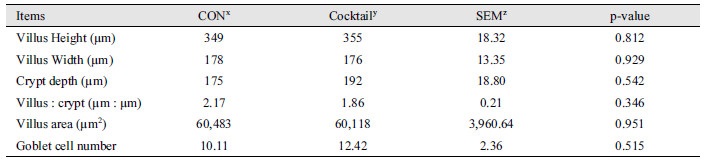
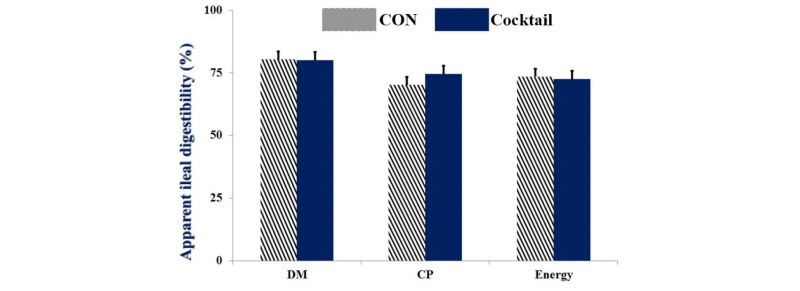
No statistical differences were observed for growth performance during the experimental period between dietary treatments (Table 2). There were no significant differences on villus height, crypt depth, and ratio of villus height to crypt depth in ileum between CON and Cocktail groups (Table 3). No statistical differences (Fig. 1 and 2) were also observed for digestibility of DM, CP, and energy between treatments.
Fig. 1.
Apparent total tract digestibility of weaned pigs fed dietary treatments. Values are means ± SEM. CON = control diet included corn and soybean meal, Cocktail = CON with 0.05% enzyme cocktail (mixture of xylanase, α-amylase, protease, β-glucanase, and pectinase). No statistical differences were observed between CON and Cocktail.
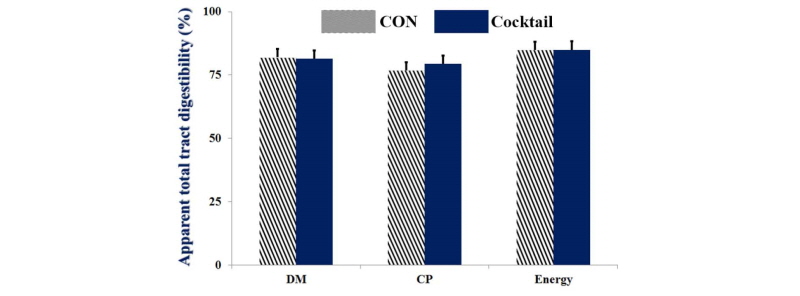
Fig. 2.
Apparent ileal digestibility of weaned pigs fed dietary treatments. Values are means ± SEM. CON = control diet included corn and soybean meal, Cocktail = CON with 0.05% enzyme cocktail (mixture of xylanase, α-amylase, protease, β-glucanase, and pectinase). No statistical differences were observed between CON and Cocktail.
Results showed that enzyme cocktail supplementation had no effects on growth performance, intestinal morphology, and nutrient digestibility. Previous studies also failed to observe positive effects of carbohydrase on growth performance and nutrient digestibility (Officer, 1995; Barrera et al., 2004; Olukosi et al., 2007). This experiment used an enzyme cocktail which was composed of xylanase, α-amylase, protease, β-glucanase, and pectinase. Xylanase was the main enzyme of the cocktail used in this research (xylanase: 4,000 units/g, α-amylase: 1,000 units/g, protease: 500 units/g, β-glucanase: 150 units/g, pectinase: 25 units/g). However, arabinoxylan in corn and soybean meal based diets was approximately 2.4% in the current research. Thus, enzyme cocktail could not digest corn and soybean meal based diets effectively.
Consequently, based on the results of the current study, weaned pigs fed diets supplemented with an enzyme cocktail did not show significant changes in growth performance, intestinal morphology, nutrient digestibility compared with those fed CON diets.