Introduction
Gamma-aminobutyric acid (GABA) is a primary inhibitory neurotransmitter of the central nervous system (CNS). It has been found in high concentrations in the brain (Dalvi and Rodgers, 2001). It controls of ingestive behavior by acting through GABA receptors and relieves the intensity of stress in human beings and animals (Shekhar et al., 2006). It was shown to function as a neurotransmitter not only in the CNS, but also in the peripheral nervous system, and to act as a local hormone or nutrition factor (Xu et al., 2000). In previous studies, Zou et al. (2009) found that the dietary supplementation of GABA can improve the growth performance of finishing pigs. Baldwin (1990) found that GABA injections can improve the Average Daily Feed Intake (ADFI) of pigs. meanwhile, the GABA was beneficial for other animals such as sheep (Girard et al.,
1985), turkey (Michael, 1991), mice (Dalvi and Rodgers, 2001), chicken (Chen et al., 2013), and sow (Fan et al., 2015a).
The inclusion of GABA in injections for mammals and avians has been studied for decades with both negative and positive results during veterinary procedures (Morteza et al., 2008; Zendehdel et al., 2009; Wang et al., 2013). However, little research has been conducted to evaluate the effects of GABA in the diet, especially in finishing pig. This study was conducted to investigate the effect of GABA on feed intake, nutrient digestibility, blood profiles, meat quality, and backfat thickness in finishing pigs.
Materials and Methods
The experiment was conducted at the swine experimental unit of Dankook University (Anseodong, Cheonan, Chungnam, Korea). The protocol for the current experiment was approved by the Animal Care and Use Committee of Dankook University. The γ-aminobutyric acid used in the current experiment was provided by a commercial company (Milae Resources ML Co., Ltd., Korea).
Animals and diets
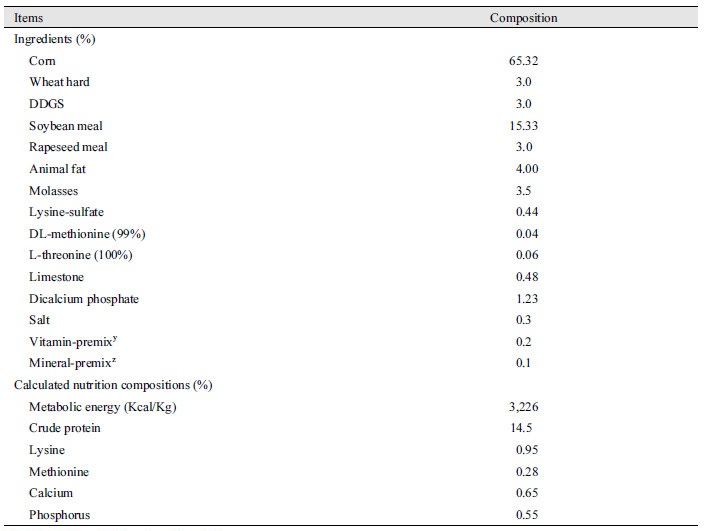
A total of 100 mixed sex pigs [(Landrace × Yorkshire) × Duroc] with initial body weight (52.03 ± 1.08 kg) were used in a 10 weeks trial. Dietary treatments consisted of 0.05% of GABA in the basal diet (GAB) and a control treatment without supplementation (CON). Each treatment consisted of 10 replications per treatment and 5 pigs (3 barrows and 2 gilts) per pen. The diets were formulated to meet or exceed the nutrient requirements recommended by NRC (2012). A corn-soybean meal-based diet was formulated as a control diet (Table 1). Pigs were housed in environmentally controlled rooms at 24 ± 1℃ and were allowed ad libitum access to feed and water throughout the experimental period, through a self-feeder and nipple drinker, respectively.
Sampling and chemical analysis
Body weight was measured individually at the beginning, middle (5th wk), and the end (10th wk) of the experimental period. Feed consumption was recorded on a pen basis during the experiment to calculate the average daily gain (ADG), average daily feed intake (ADFI), and gain/feed ratio (G : F) (Jeong et al., 2017; Yun et al., 2017).
Chromium oxide (Cr2O3) was added to the diet at 0.2% of the diet as an indigestible marker for 7 days prior to fecal collection on weeks 5 and 10 to calculate dry matter (DM), nitrogen (N) and energy. Fecal grab samples were collected randomly from at least two pigs in each pen (1 gilt and 1 barrow) at weeks 6 and 12 of the experiment. All feed and fecal samples were immediately stored at - 20℃ until analysis. Feces samples was dried 72 hours in the dryer at 60℃ and finely ground to allow for passage through a 1 mm screen for analyzing apparent total tract digestibility of dry matter (DM) (AOAC method 930.15), nitrogen (AOAC method 990.03) and energy (using a bomb colorimeter, Parr 61.00; Parr Instruments Co. Moline, IL), following the procedures outlined by AOAC (2000). Chromium was analyzed via UV absorption spectrophotometry (Shimadzu, UV-1201, Kyoto, Japan), following the method described by (Williams et al., 1962). Gross energy in the feces was also determined using a calorimeter (Mode 1241, Parr Instrument Co. USA). The digestibility was then calculated using the following formula: digestibility (%) = {1 - [(Nf × Cd) / (Nd × Cf)]} × 100, where Nf = nutrient concentration in feces (% DM), Cd = chromium concentration in diet (% DM), Nd = nutrient concentration in diet (% DM), and Cf = chromium concentration in feces (% DM) (Stein et al., 2006).
Two pigs were selected randomly from each pen and bled via jugular venipuncture at the beginning of the experiment. The same pigs were also bled on weeks 5 and 10 of the experiment. Blood samples were collected into 5 mL vacuum tubes with K3EDTA (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ, USA) and placed on ice until analysis. Whole blood samples were subsequently centrifuged for 15 min at 3,000 × g at 4℃ and the harvested serum was used to determine blood profiles. The concentration of cortisol, epinephrine, norepinephrine, glucose and erythropoietin was determined using an automatic biochemistry analyzer (HITACHI 747, Tokyo, Japan).
At the end of the experiment, the pigs were slaughtered at a local commercial slaughterhouse. After chilling at 2℃ for at least 24 h, two 2.54 cm thick longissimus muscle (LM) samples were removed at the 10th rib (right side of the carcass). Meat color, sensory evaluation, pH and water holding capacity (WHC) were then measured. Meat color of the longissimus muscle as lightness (L*), redness (a*), and yellowness (b*) was determined using a Minolta Chromameter (CR-210, Minolta, Japan) to evaluate the freshly cut surface after 30 min of blooming at 4℃. Sensory evaluation was conducted by 6 trained panelists to evaluate the color, firmness and marbling of fresh loin samples using a three-point assessment scheme according to the procedures established by the NPPC (1991). The pH values were measured directly at 3 points of the LM using a combined pH electrode (Nwkbinar, pH, K-21, Landsberg, Germany). The water holding capacity (WHC) was measured according to the methods described by (Kauffman et al., 1986). Briefly, a 0.3 g sample was pressed at 3,000 psi for 3 min on a 125 mm diameter piece of filter paper. The areas of the pressed sample and the expressed moisture were delineated and then determined using a digitizing area-line sensor (MT-10S; M.T. Precision Co. Ltd. Tokyo, Japan). The ratio of water: meat area was calculated, giving a measure of WHC (a smaller ratio indicates increased WHC). The longissimus muscle (LM) area was measured by tracing the LM surface at the 10th rib, which was conducted using the aforementioned digitizing area-line sensor. Drip loss was measured using approximately 2 g of meat sample at d 1, 3, 5 and 7 after slaughter according to the plastic bag method described by (Honikel et al., 1986),cook loss was determined as described by (Sullivan et al., 2007). The backfat of pigs was measured 6 cm off the midline at the 10th rib using a real-time ultrasound instrument (Piglot 105, SFK Technology, Herlev, Denmark).
Statistical analysis
Data were statistically analyzed by ANOVA, using general linear model (GLM) procedure of the SAS program (SAS Inst. Inc. Cary, NC, USA), for a randomized complete block design. Mean values and standard error of means (SEM) are reported. Statements of statistical significance are based on p < 0.05.
Results
Growth performance
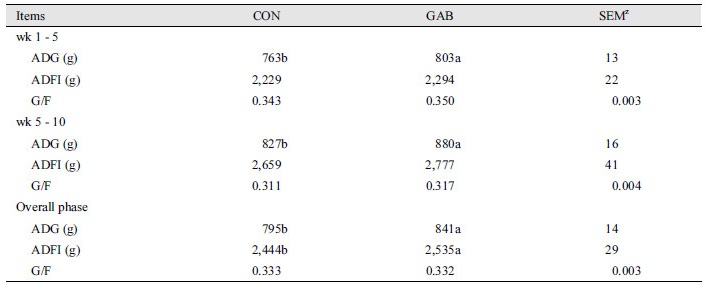
Table 2 shows, the effect of dietary supplement of GABA on the growth performance in finishing pigs. During weeks 1 - 6, 6 - 12, and the overall experimental period, ADG of the pigs in GAB treatment group was higher (p < 0.05) than that of pigs in the CON group. The ADFI of the pigs of was increased in GAB treatment group compared with CON treatment group for the overall experimental period.
Nutrient digestibility
Table 3 shows the digestibility of nutrient in finishing pigs. DM digestibility was greater in GAB treatment than CON treatment (p < 0.05). The GABA supplemented diet did not affect the N and energy digestibility during experimental period (p > 0.05).
Blood profiles
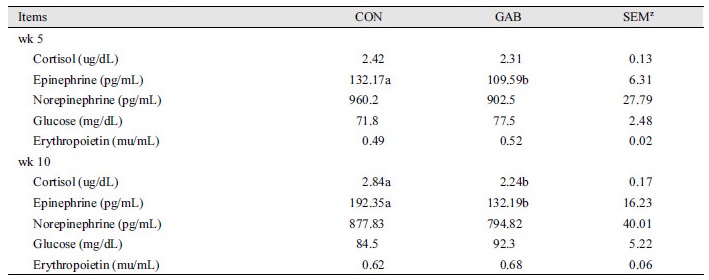
Table 4 shows, the effect of dietary supplement of GABA on blood profiles throughout experiment. Epinephrine was decreased in GAB treatment compared with CON at 5th and 10th weeks (p < 0.05). Also, cortisol was lower than CON treatment at 10th week in GAB treatment (p < 0.05). There was no effect on norepinephrine, glucose, and erythropoietin during the experiment (p > 0.05).
Meat quality
Table 5 shows the effects of dietary supplementation of GABA on meat quality throughout the experiment. The activity of meat color, sensory evaluation, cooking loss, drip loss, pH, loin muscle area and water holding capacity did not change during the experimental period (p > 0.05).
|
Table 5. Effect of dietary supplementation of GABA on meat quality in finishing pigs. 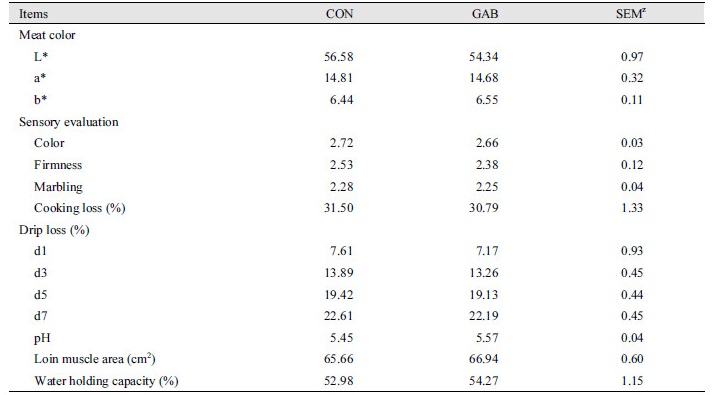
|
|
|
CON, basal diet; GAB, basal diet + 0.05% GABA. zStandard error of means. |
|
Backfat thickness
Table 6 shows the effects of dietary supplementation of GABA on backfat thickness. As compared with CON, the GABA supplementation treatment had no effect during experiment period (p > 0.05).
Discussion
It has been reported that dietary GABA supplementation had widely varying effects on animals and plays a great role in performance, appetite regulation, improving nutrient utilization efficiency, and alleviating heat stress in the body (Zhang et al., 2012; Chen et al., 2013).
Zou et al. (2009) reported that dietary supplementation of GABA can effectively improve growth performance, enhance antioxidation performance, and thyroid function of growth-finishing swine during hot summers when they were added into diet at the optimal dosage of 10 mg/kg.
In the present experiment, the dietary supplementation of GABA caused an increase in feed intake during the overall experimental period (Table 2). Also, Table 2 shows that GABA improved ADG in all experiment phases. Supplementation of GABA improved performance including ADG, FI and feed conversion rate of growing pigs when they were fed the basal diet supplemented with 100 mg/kg GABA (Fan et al., 2015a). Fan et al. (2015b) state that the optimal supplementation level of GABA is 300 mg/kg of the diet, and had a significant and direct effect on the sow including ADFI and litter weight gain of piglets during the whole lactation period.
Previous results indicated that GABA reduced serum leptin level and promoted FI through activating neuropeptide Y neurons (Besheer et al., 2004). Li et al. (2015) showed that plasma growth hormone and neuropeptide Y concentrations were significantly increased in weaned pigs by GABA supplementation. Some reports show that dietary supplement of GABA could regulate endocrine systems, enhance the immunity, and the antioxidant capacity of pigeon (Huang et al., 2011). Other results show that dietary GABA supplementation had significantly increased the activities of glutathione peroxidase and superoxide dismutase and also improved serum calcium, phosphorus, kalium, chloride glucose concentrations. Serum total protein, albumin, urea nitrogen concentrations showed improvements in finishing hogs (Hu et al., 2008). However, in this study, the concentration of epinephrine and cortisol were decreased in GAB treatment during the experimental period (Table 4). Similar results could not be confirmed from previous studies, so more research is needed to prove this result is reliable.
Thus, we found that dietary supplement of GABA could improve the digestibility of DM. It is possible that GABA is a modulator of peristaltic activity via the regulation of acetylcholine release from cholinergic neurons through interaction with GABAA or GABAB receptors (Auteri et al., 2014). In addition, GABA could cause transient relaxations of the longitudinal and circular muscle of the colon and transient constrictions followed by relaxation of the muscle of the ileum (Krantis et al., 1980).