Introduction
The term medium chain triglycerides (MCTs) obtained by the hydrolysis of coconut oil followed by the fractionation of the fatty acids refers to mixed triglycerides of saturated fatty acids with a chain length of 6 – 10 carbons. Compared to long-chain triglycerides (LCT), MCT displays some specific physicochemical and biological characteristics. MCT provides polka dot grouper Chromileptes altevelis with a more readily utilizable source of energy than long-chain fatty acid (LCFA), and significantly elevated the plasma cholesterol level of chickens (Fisher and Kaunitz, 1964; Mabayo et al., 1992; Akiba et al., 1993; Smith et al., 2005).
MCTs are currently used in clinical nutrition as energy-yielding substrates, and have been advocated for three decades as a useful mean for body weight reduction (Bach et al., 1996). Also, MCT is widely used in obese nondiabetic humans for inducing fat loss and decreasing blood levels of ketone bodies (Marten et al., 2006b; Han et al., 2007). MCTs reduce fat mass, through down-regulation of adipogenic genes as well as peroxisome proliferator activated receptor-γ. MCTs improved several features contributing to enhanced insulin sensitivity. Under certain in vitro conditions, MCTs exert proinflammatory effects, but in vivo MCTs may reduce intestinal injury and protect from hepatotoxicity (Marten et al., 2006a; Leveille et al., 2016).
The objective of this study was to investigate the effect of different phase level of MCT on growth performance, excreta microflora and blood profiles in broilers for formulating cost effective feeds.
Materials and Methods
All animal-based procedures were in accordance with the Guidelines for the Care and Use of Experimental Animals of Dankook University.
Experimental material
The MCT (AveMix) was prepared and supplied in the form of powder by a commercial company (Aveve Group Co. Ltd, Leuven, Belgium). The composition of the final product contained 55% MCT oil (C6: 50%, C8: 50%) and Silica carrier 45%.
Experimental birds and diets
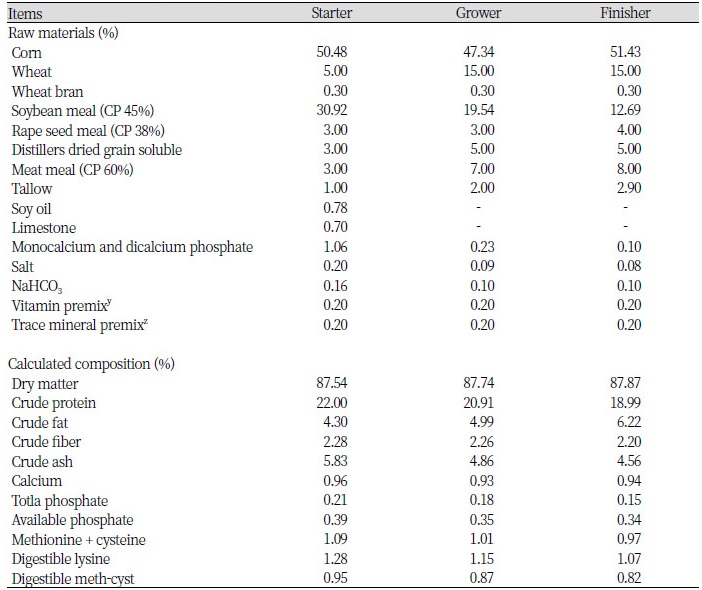
A total of 450 ROSS 308 mixed-sex broilers with an average initial body weight (BW) 49 ± 0.79 g (1 d of age) were used in this trial. They were randomly assigned to 3 treatments, with 15 birds of 10 replications in each treatment of following: CON (Basal diet); MCT1 (Starter, Grower, Finisher: Basal diet + 0.1%, 0.075%, 0.05% of MCT); MCT2 (Starter, Grower, Finisher: Basal diet + 0.1%, 0.1%, 0.1% of MCT). The experiment was conducted in 3 phases consisting of starter phase (from d 1 to 7), grower phase (from d 8 to 21) and finisher phase (from d 22 to 38). Ingredients and calculated nutrient composition of basal diets from starter to finisher phase were presented in Table 1. Broilers were raised in a temperature-controlled room with three floors of stainless steel pens of identical size (1.75 × 1.55 m2). Room temperature began at 33°C from day 1 to day 3 and was reduced gradually to 24°C by the end of the experiment. Artificial light was provided 24 h/d by the use of fluorescent lights. The relative humidity was around 60%. Birds had free access to feed and water throughout the study.
Sampling and Chemistry analysis
The broiler chicks were measured BW and feed intake (FI) was recorded on d 1, 21 and 28. At the end of the experiment, 30 broilers were randomly selected from each treatment and blood samples were collected from the wing vein into a sterile syringe. After collecting and blood samples were transferred into vacuum tubes containing K3EDTA (Becton, Dickinson, Franklin Lakes, NJ). Blood samples were centrifuged (3 000 × g) for 15 min at 4°C to obtain serum. The concentrations of white blood cell (WBC) and lymphocyte percentage were analysed by using an automatic blood analyzer (ADVID 120, Bayer, USA). Immunoglobulin G was analyzed by using an automatic biochemistry analyzer (HITACHI 747, Tokyo, Japan).
Fecal samples freshed were collected at the end of experiment (4 weeks). Then serial dilution (10-4 to 10-7) of digesta samples was made using anaerobic diluents and placed on MacConkey agar plates (Difco Laboratories, Detroit, MI) and Lactobacilli medium III agar plates (Medium 638; DSMZ, Braunschweig, Germany) to isolate the Escherichia coli (E.coil) and Lactobacillus, respectively. The Lactobacilli medium III agar plates were then incubated for 48 h at 39℃ under anaerobic conditions. The MacConkey agar plates were incubated for 24 h at 37℃. The E.coli and Lactobacillus colonies were counted immediately after removal from the incubator.
Statistical analysis
All experimental data were analyzed by ANOVA using the general linear model (GLM) procedure (SAS Inst. Inc., Cary, NC) with the cage being defined as the experimental unit. Differences among treatments were separated by Duncan’s multiple range test. The results were expressed as the least squares means and standard error. A criterion α level of p < 0.05 was used to determine statistical significance.
Results and Discussion
Growth performance
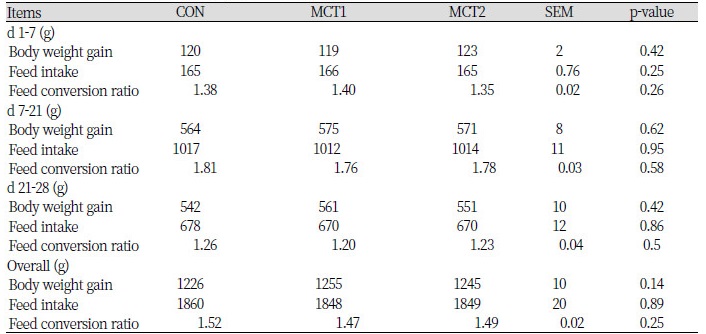
The effects of MCT on growth performance were shown in Table 2. These results indicated the supplementing the diets with different phase level of MCT did not have significant impact on BWG, FI and feed conversion ratio (FCR) during the overall experimental period.
MCTs are a unique form of dietary fat that impart a wide range of positive health benefits. There are a few studies about MCTs on broilers. Chicken dietary supplementing MCT to the chick diet would improve body weight gain and protein utilisation (Yen et al., 2015b). The MCTs has been extensively studied in other animals. Some studies indicated that the pigs fed MCT diets had higher average daily gain (ADG), improved the apparent total tract digestibility (energy, dry matter and nitrogen) and gain-to-feed ratio, also decreased coliforms counts in colon and rectum content (Mabayo et al., 1993; Gatlin et al., 2002; Hong et al., 2012; Yen et al., 2015a). Hernández and Pluske (2008) 's results indicated that there was no difference between experimental diets by adding MCT on pig performance. Nielsen et al., (2005) and Asdari et al., (2014) reported that the addition of MCT to the diet had different effects on growth performance and protein content of the fish. In this study, we found that the BWG was increased 2.3% and FCR were decreased 0.6% in broiler fed MCT1 in the overall experiment phase. Thus the addition of 0.1% MCT seems to have greater ADG than CON, although there was no statistical difference. Furuse et al., (1993)’s report demonstrated that chicks preferred the LCT-supplemented diet compared with either of the diets containing MCT. Similar results were found by other researcher that it was obtained that given treatments effective on feed intake, weight gain and the feed conversion were not significant when given the basal diet plus 0.1, 0.15 or 0.2% for period 1 – 21 day of age, 0.15% for period 21 – 36 day of age and 0.1% for period 36 – 42 day of age from MCFA (Shahram et al.2013). In our previous study, we used one hundred and twenty weaning pigs to evaluate the effect of MCT on growth performance, apparent total tract digestibility (ATTD) of nutrients and blood profile. Results indicated that the addition of MCT in the weaning pigs diet could improve the ADG and digestibility during the earlier period (first 2 wks), but had little effect on the blood characteristics (Hong et al., 2012).
Blood characteristics
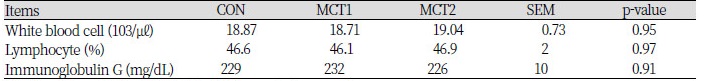
Table 3. showed the effect of MCT on blood profiles throughout the experiment. The results demonstrated that the concentration of white blood cells, lymphocyte and IgG, were unaffected by MCT supplementation in this study.
Changes in blood parameters reflect the body's metabolic changes. IgG in the blood is mainly produced by the B cells into plasma cells after secretion, when the body is stimulated by the antigen, the blood levels of antibodies, IgG levels will be increased. IgG can be combined with the specific antigen, causing immune response to protect the body. its level of content to a certain extent reflects the level of the body resistance. MCT added in dietary had no significant effect on blood profile and excreta microflora in broiler. Hernández and Pluske (2008) and Hong et al., (2012) also reported that no effect was observed on the blood profiles (WBC, IgG and lymphocyte concentration) measured.
Excreta microflora
The effect of MCT on excreta microflora in broiler was shown in Table 4. The results demonstrated that no significant effect was observed in Lactobacillus and E.coil throughout the entire experimental period.
Any effects were not found on excreta microflora in broiler. With similar study, Zentek et al., (2013)’s research demonstrated that the MCFA induced only minor changes in the gastrointestinal microbiota but increased cell counts for the Escherichia-Hafnia-Shigella group in the jejunum and the clostridial cluster XIVa in the colon digesta. Also, Similar results were reported by Shahram et al. (2013) that there was no statistically significance on microbial population (lactobacilus) in MCFA diets. However, Yen et al., (2015a) reported that supplementation with MCT in diet could improve the intestinal microbial environment and the feed utilization efficiency of newly weaned pigs. This inconsistency maybe due to different dosage and composition of MCTs and experimental conditions used in these investigations. In summary, we suggested that MCTs could be used as an alternative to growth promotion additive. Moreover, it is also suggested that further research should be performed to evaluate the effect of the different level of MCTs.