Introduction
The aging process is widely used for the enhancement of beef palatability, including tenderness and flavor (Sitz et al., 2006; Lee et al., 2018). There are two types of aging: Wet and dry aging. In wet aging, meat is vacuum-packed and aged under refrigeration (Smith et al., 2008). Dry aging refers to the exposure of meat to air at controlled temperature, relative humidity, or/and air flow. Dry-aged beef is reported to have a more “beefy” and “brown-roasted” flavor compared to wet-aged or non-aged beef (Warren and Kastner, 1992). However, dry aging in beef leads to higher weight loss due to greater water and trim loss compared to wet-aged or non-aged beef (Parrish et al., 1991). Despite the high cost of the process, the application of dry aging in beef has rapidly increased in popularity recently due to the resulting enhanced palatability and unique flavor.
Among consumers who prefer dry-aged meat, there is a particular demand for safety while maintaining the characteristic flavor attributes, because of the high possibility of contamination in dry-aged meat during the aging or/and trimming process. Vacuum packaging, commonly used for the distribution or sale of meat, is utilized to control microbial growth in meat. Wrap packaging with oxygen-permeable polyvinyl chloride (PVC) film is also widely used for fresh beef during retail display (Jayasingh et al., 2001). The optimal storage period for wrap- or vacuum-packaged meat to maintain meat quality, including physicochemical and sensory properties, depends on the storage temperature (Jakobson and Bertelsen, 2000). It is especially important for dry-aged meat to determine the optimal storage conditions (temperature and storage period) for different packaging methods to maintain its inherent sensory characteristics.
To our knowledge, there has been no study determining the microbial, physicochemical, and sensory properties of differently packaged beef after dry aging with subsequent storage at different temperatures. In other words, it is necessary to determine the shelf-life of dry-aged beef in common storage conditions such that there is no adverse effect on meat quality. Doing so will allow the practical needs of producers and distributors in the rapidly growing dry-aged meat market to be met. Therefore, the objective of this study was to investigate the changes in microbial, physicochemical, and sensory properties of dry-aged beef resulting from different packaging methods and storage temperatures during extended storage times.
Materials and Methods
Raw materials and dry aging
A total of 28 sirloins (approximately 54-month-old Hanwoo cows; quality grade 2) were collected and transported in an iced condition (4°C) to Korea Institute for Animal Products Quality Evaluation (Sejong, Korea). Initial pH was measured for all samples prior to the dry aging process. Ten sirloins were designated as non-aged controls, and the other 18 (n = 6 for each of three treatments, described below) were dry aged at 2 ± 1°C (75% relative humidity) for 28 days. The non-aged control samples were frozen immediately. After 28 days of dry aging, the dried surfaces (crusts) of the dry-aged beef were trimmed off, and the beef was packed and stored using the following packaging methods and temperatures for 21 days: 1) overwrapped with oxygen-permeable PVC film and stored at 3 ± 2°C; 2) vacuum-packaged and stored at 3 ± 2°C; 3) vacuum-packaged and stored at -23 ± 2°C. Vacuum packaging was carried out using polyethylene bags (O2 permeability 2.3 mL/m2/d at 38°C; HFV-600L, Hankook Fujee Co., Ltd., Siheung, Korea). Meat samples were analyzed at days 0, 7, 14, and 21 of storage after the completion of dry aging and packaging.
Total aerobic bacteria (TAB)
Meat samples (5 g) were blended with sterile saline (45 mL, 0.85%) for 2 min using a BagMixer® 400 P blender (Interscience, St. Nom la Bretèche, France), and diluted (Yoon et al., 2017). Each sample (100 μL) was spread on plate count agar (Difco Laboratories, Detroit, MI, USA) to inspect TAB. The agar plates for TAB and were incubated at 37°C for 48 h. After incubation, microbial counts were calculated and expressed as log CFU/g sample.
2-Thiobarbituric acid-reactive substances (TBARS) value
Each sample (3.0 g) was homogenized (T25 Basic, Ika works, Staufen, Germany) in 9 mL of distilled water and 50 μL of butylated hydroxyl toluene as previously described (Khan et al., 2016). The homogenate (1 mL) was mixed with 2-thiobarbituric acid/trichloroacetic acid solution (2 mL). The mixture was heated at 90°C for 30 min in a water bath, cooled, and centrifuged at 2,090 × g (Continent 512R, Hanil Co., Ltd., Incheon, Korea). The absorbance of the supernatant was measured at 532 nm using a spectrophotometer (X-ma 3100; Human Co. Ltd., Seoul, Korea) and 2-thiobarbituric acid-reactive substances (TBARS) value [mg malondialdehyde/kg meat sample] was calculated using a standard curve.
Instrumental color
The samples were bloomed for 30 min and CIE color values (L*, a*, and b*) were determined using a spectrophotometer (CR400; Konica Minolta Censing Inc., Osaka, Japan). Before color measurement, the spectrophotometer was calibrated with a standard tile (L* = 97.74, a* = -0.06, b* = 1.76). Each sample was measured three times, and the average value was used as one replicate. Chroma [(a*2 + b*2)] and hue angle [tan-1(b* / a*)] were derived from CIE L*, a*, and b* values.
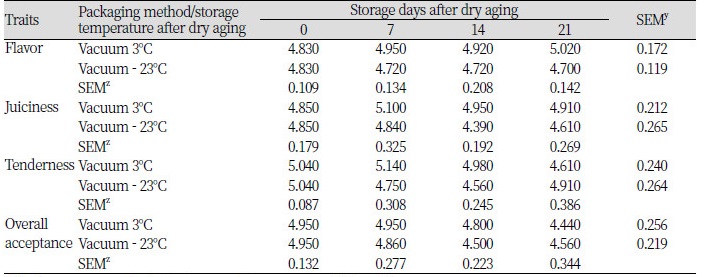
Sensory evaluation
Statistical analysis
The experimental design was a randomized incomplete block design using trial as block. The non-aged meat (control, n = 10) and 3 treatments (n = 6/treatment) were assigned and a model was analyzed with fixed effects (packaging and temperature) and random effects (carcass and side of carcass). An interaction effect between storage temperature and storage time was considered for color values (L*, a*, b*, chroma value, and hue angle) and lipid oxidation. A general linear model was generated using SAS 9.3 (SAS Institute Inc., Cary, NC, USA), and mean values ± standard error of the mean (SEM) reported. Significant differences between the mean values were determined based on the Student-Newman-Keuls multiple comparison test at a level of p < 0.05.
Results and Discussion
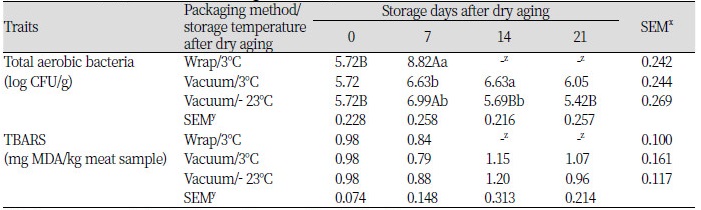
Total aerobic bacteria
There was no significant difference in TAB counts between non-aged (control, 5.83 log CFU/g) and dry-aged beef (5.72 log CFU/g). The observation might be due to prevention of microbial penetration into the meat by crust formation during dry aging. This is consistent with the previously reported finding that dry aging for 28 days in beef strip loin did not affect the number of total aerobic bacteria (p > 0.05) (DeGeer et al., 2006). As presented in Table 1, the TAB counts of dry-aged beef with wrap packaging reached approximately 8.8 log CFU/g at day 7 of refrigerated storage (3 ± 2°C), indicating meat spoilage (Sofos et al., 2000). In the vacuum-packaged dry-aged beef, TAB counts were below 7 log CFU/g for the entire storage period regardless of storage temperature. This result could be because the application of vacuum packaging eliminated oxygen, led to retardation of aerobic bacteria, and reduced the growth rate of anaerobic and facultative bacteria (Bellés et al., 2017). This result indicates that the application of vacuum packaging to dry-aged beef can control microbial growth up to 21 days of storage at both 3°C and - 23°C.
2-Thiobarbituric acid-reactive substances (TBARS) value
Dry aging of beef led to an increase in lipid oxidation compared with non-aged control (p < 0.05; data not shown). This result may be due to the fact that the beef was exposed to air during dry aging. Higher lipid oxidation has previously been observed in dry-aged beef with the same quality grade used in this study compared with non-aged beef (Lee et al., 2015). The packaging method, storage temperature, and storage periods did not influence lipid oxidation in dry-aged beef, with TBARS values ranging from 0.84 to 1.20 (p > 0.05; Table 1). The TBARS values showed that there was no negative effect on meat quality as values remained below 1.2, which is considered as a threshold value for detection of “off” flavor in meat (Younathan and Watts, 1959). A previous study reported that vacuum packaging of lamb meat led to effective reduction in lipid oxidation at 2 - 4°C after storage for 28 days (Fernandes et al., 2014). There was no interaction between storage temperature and storage periods in this study.
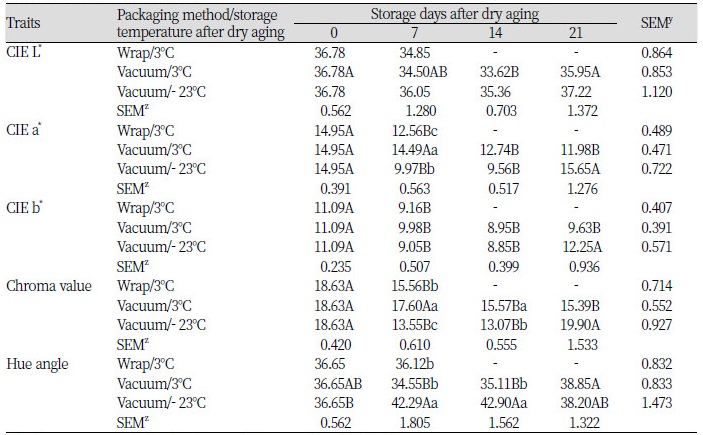
Instrumental color
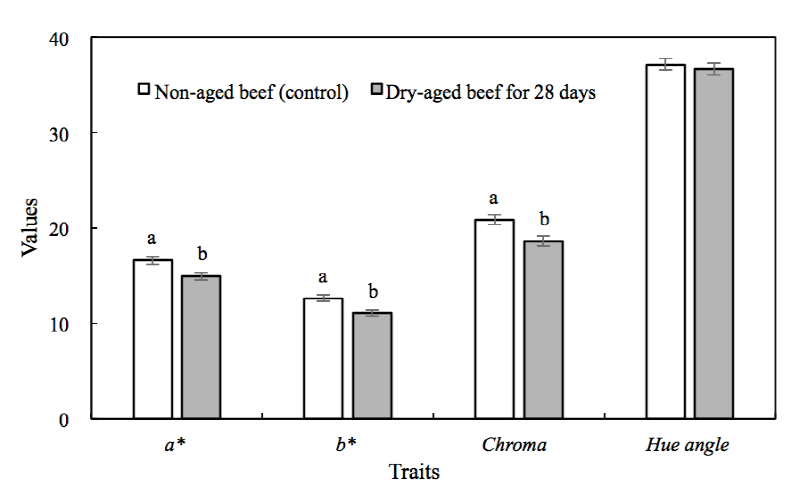
Different L*, a*, and b* values were observed depending on dry aging, packaging method, and storage temperature (Table 2). Dry aging led to significantly lower a*, b*, and chroma values in beef sirloin (Fig. 1). The L* value (data not shown) and hue angle (Fig. 1) of the beef were not affected by dry aging (p > 0.05 compared with non-aged control; Fig. 1). The a* value of the beef was affected by packaging method at day 7 of storage; vacuum-packaged dry-aged beef had higher a* values than wrap-packaged dry-aged beef (p< 0.05). According to Ledward (1985), vacuum packed meat during storage tended to have relatively higha* values as helping to maintain metmyoglobin reducing activity. Significant differences in a* and b* values were observed for each packaging method and storage temperature. During refrigerated storage, vacuum-packaged dry-aged beef maintained a* and chroma values up to day 7, but significant decrease in a* values were observed thereafter until day 21. During frozen storage, a* values of vacuum-packaged dry-aged beef decreased until day 14. Dry-aged beef with vacuum packaging showed significantly higher a* and chroma values at day 21 of frozen storage compared with day 7 and day 14. In other words, for dry-aged beef under vacuum packaging, higher color stability was observed with refrigerated storage compared to frozen storage over a short-term storage period (7 days). Moreover, an interaction was observed between storage temperature and storage periods for a*, b*, and chroma values.
Conclusion
The results of this study indicate that dry-aged beef can be stored without, or with minimal, microbial growth and quality deterioration for different periods depending on the packaging method and storage temperature. With respect to microbial growth, it is recommended that dry-aged beef with wrap packaging stored in refrigerated conditions should be consumed as quickly as possible. Vacuum packaging can extend the color stability of displayed beef after dry aging over short periods (less than 14 days) of refrigerated storage without problems related to microbial spoilage, lipid oxidation, or sensory quality. For long-term storage, the results of this study suggest that dry-aged beef should be frozen, as freezing can extend the color stability up to day 21 of storage without adverse effects on hygienic or meat quality aspects of dry-aged beef.