Introduction
It is well known that weaning pigs often face post-weaning challenges, such as diarrhea, low feed intake, body weight (BW) loss (Pluske et al., 1996). These affect the health of weaning pigs, thereby affect the economic value of pigs. Interest in the use of yeast culture (YC) as feed supplements for pigs has been increased markedly in recent years. Fully fermented YC is a dried product containing yeast and various metabolites of yeast fermentation. , YC has been used in feeding dairy cows to improve milk production (Robinson and Garrett, 1999). Recently, a report showed that feeding YC to gestating and lactating sows improved litter weight gain (Kim et al., 2008). Mathew et al. (1998) and Bontempo et al. (2006) showed that feeding YC to weaning pigs improve the average daily gain (ADG) and average daily feed intake (ADFI). A commonly used yeast culture is the saccharomyces cerevisiae fermentation product. saccharomyces cerevisiae is one kind of yeast which is used to ferment yeast culture (Minervini et al., 2012). A saccharomyces cerevisiae fermentation product is produced during fermentation of an unmodified strain of saccharomyces cerevisiae, including the products of fermentation, residual yeast cells, yeast cell wall fragments, and the media used during fermentation (Shen et al., 2011). Some research indicated that supplementation with YC enhanced the growth performance, nutrient digestion, reproductive performance of pigs, and so on (Vander Peet-Schwering et al., 2007; Kim et al., 2008; Kim et al., 2010). Yeast culture (saccharomyces cerevisiae) has been shown to improve total tract nutrient digestibility of nursery pigs (Shen et al., 2009). However, another report showed that feeding yeast culture to animals had no significance in growth performance (Martin et al., 1992). Thus this study was conducted to determine the effects of YC (saccharomyces cerevisiae) on growth performance, fecal score, and nutrient digestibility of weaning pigs.
Materials and Methods
All animals received human care as outlined in the guide for the care, and use of experimental animals (Animal Care Committee, Dankook University, Korea).
Animals and diets
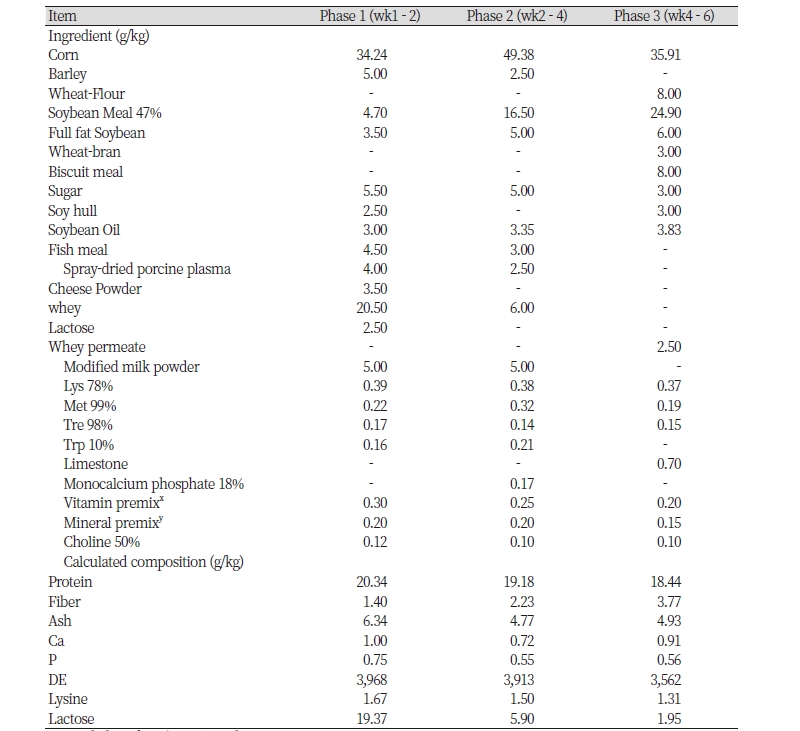
A total of 50 crossed healthy weaning pigs [(Yorkshire × Landrace) × Duroc] with an average BW of 7.46 ± 1.60 kg (28 d of age) were used in a 6 weeks experiment that was divided into 3 phases of 2 week each. Pigs were randomly allotted to 1 of 2 experimental diets according to initial BW in a randomly complete block design. There were 5 replicated pens per treatment with 5 pigs per pen. Dietary treatments were 1) CON: basal diet, 2) CON + 0.50% YC. All diets were formulated to meet or exceed the recommendation of for weaning pigs, and were fed in a mash form (). The product has been evaluated in this trial was yeast culture (XPC, Diamond V original XPCTM Yeast culture, Cedar Rapids, IA, USA). All pigs were housed in an environmentally controlled nursery room. The stainless steel pens were 0.5 m × 0.6 m × 2.0 m with a slatted plastic floor. Each pen was provided with a stainless steel feeder and a nipple waterer that allowed ad libitum access to feed and water throughout the experiment. Ventilation was provided by a mechanical system and lighting was automatically regulated to provide 12 h of artificial light per day. The ambient temperature within the room was approximately 30℃at the start of the experiment and decreased by 1℃ each week.
Chemical analysis
Feed and fecal samples were ground to pass through a 1mm screen, after which were analyzed for dry matter (DM) (method 934.01; AOAC, 2000), crude protein (CP, method 990.03; AOAC, 2000), crude fat (method 920.39; AOAC, 1995). Nitrogen (N) was determined by the machine (Kjeltec 2300 Nitrogen Analyzer; Foss Tecator AB, Hoeganaes Sweden), and CP was calculated as N × 6.25. Energy was analyzed by oxygen bomb calorimeter (Parr 1600 Instrument Co., Moline, IL, USA). Chromium was analyzed by UV absorption spectrophotometry (Shimadzu UV-1201; Shimadzu, Kyoto, Japan) following the method described by Williams et al. (1962). Digestibility was calculated using chrome oxide as an indigestible marker.
Sampling and measurements
Individual pig BW, and feed refusals were recorded on Wk 2, 4, and 6 to calculate ADG, ADFI, and growth efficiency (gain/feed ratio, G/F). On Wk 6, the experimental diets were supplemented with 0.2% chromium oxide (Cr2O3) as a non-digestible marker to calculate the apparent nutrient digestibility. After 7 d, fresh fecal samples were obtained from each pig by anal massage. Representative samples were stored at - 20℃ until analyzed. Before chemical analysis, the fecal samples were thawed, and dried at 60℃ for 72 h, after which they were finely ground to a size that could pass through a 1 mm screen. All feed and fecal samples were then analyzed for DM, N, and energy (E) as described before, and using the following formula: digestibility (%) = [1 – {(Nf × Cd)/(Nd × Cf)}] × 100, where, Nf = nutrient concentration in feces (% DM), Nd = nutrient concentration in diet (% DM), Cd = chromium concentration in diet (% DM), and Cf = chromium concentration in feces (% DM) (Zhang et al., 2018).The incidence of diarrhea in weaning pigs was observed, and recorded 3 times per day throughout the study. In order to assess the severity of diarrhea, feces from each pen were scored according to the method of . In brief, the scores were as follows: 1 (hard, dry pellets in a small, hard mass); 2 (hard, formed stool that remains firm, and soft); 3 (soft, formed, and moist stool that retains its shape); 4 (soft, unformed stool that assumes the shape of the container); and 5 (watery, liquid stool that can be poured). A cumulative diarrhea score (DS) per diet, and day was then assessed ().
Statistical Analysis
The data were analyzed using the General Linear Model (GLM) procedures of SAS (SAS, 1996), and significant differences among the means were determined using Duncan’s Multiple Range Test method (Duncan, 1955), with p < 0.05 indicating significance. Probability values less than 0.10 were used as the criterion for a tendency.
Results
Growth performance
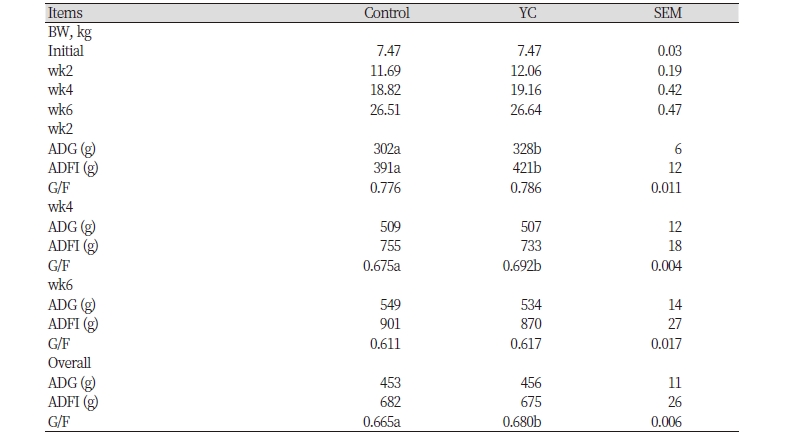
During phase 1, ADG, ADFI were significantly increased (p < 0.05) in weaning pigs fed yeast culture supplementation diets compared with weaning pigs fed CON diet (Table 2). During phase 3 and overall, G/F was significantly increased (p < 0.05) in pigs fed YC supplemented diets than CON diet. BW, ADG, and ADFI in phase 2, phase 3, and overall, G/F in phase 1 and phase 3 were not affected (p > 0.05) by dietary treatment during all phases of the experiment (Table 2). During the whole experiment, G/F in fed YC supplementation diets was increased compared with CON diet in numerical value.
Fecal score
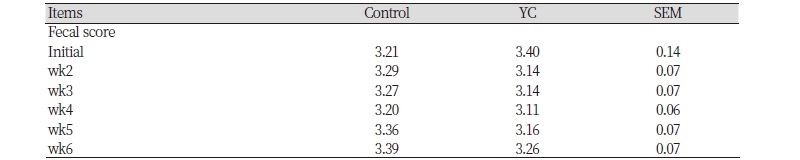
During initial, Wk 2, 3, 4, 5, and 6, there was no significant effect (p > 0.05) with YC diet on fecal score compared with CON diet of weaning pigs (Table 3).
Nutrient Digestibility
There was no significant difference in nutrient digestibility (p > 0.05) between CON and YC treatments (Table 4), however the DW, nitrogen, and Edigestibility in pigs fed YC diet were higher in numerical value than pigs fed CON diet.
Discussion
YC is rich in nutrients and growth factors. Many studies have shown that YC improved the growth performance of nursery pigs (Shen et al., 2009). Various yeast-related feed supplements were defined by the Association of American Feed Control Officials (2009). Active dry yeast could be considered as one kind of probiotics. Because it has been dried and contained at least 15 × 109 live yeast cells per gram. Yeast culture is the dried product of yeast and the media to preserve the fermenting activity of yeast, as well as the metabolites from yeast fermentation. It increased our attention to this interesting feed additive because of the growth-promoting and potential antibiotic alternative properties of yeast culture product (Price et al., 2010). Supplementing YC diets was contributed to growth of animals (Trckova et al., 2014). Some reports showed the positively increased growth performance with YC diet in weaning pigs (Mathew et al., 1998; Bontempo et al., 2006; Canibe et al, 2007). In previous studies, YC products increased ADFI and ADG (Van der Peet-Schwering et al., 2007; Shen et al., 2009), and improved the feed efficiency of pigs (Van der Peet-Schwering et al., 2007). Van Krimpen and Binnendijk (2001) showed that piglets fed diets supplemented with AGP (40 mg/kg of avilamycin) or live yeast cells (saccharomyces cerevisiae CS47) performed better than piglets fed the negative control diet. Chen et al., (2017) reported that the YC (saccharomyces cerevisiae) could positively affect BW and ADFI of broiler chickens. These results were in line with our study in the weaning pigs fed YC diets that had significant higher ADG, ADFI in phase 1 and G/F in phase 3 and overall than weaning pigs fed the basal diets. However, our findings were not exactly consistent with other people's research. Body weight, ADG, ADFI in phase 2, phase 3, and overall, G/F in phase 1 and phase 3 were not affected by dietary treatment during all phases of the experiment. In a study by Kornegay et al. (1995), the performance of piglets was not improved by supplementing the diet with YC, whereas in a study by Mathew et al. (1998), post-weaning performance did improve. Different possibilities might explain these discrepancies, such as the environment, the diet and so on. In our study, dry matter, nitrogen and energy in YC treatment though not significant had numerically higher value than control. In addition, the fecal score of weaning pigs during all phases of the experiment were not affected. It may possibility explains that YC stimulates the immune system. YC triggers the interaction between the intestinal immune response and the immune system. Energy flows in the direction of the immune function not only the growth performance. It is consistent with other authors. Stimulating the immune system, maintaining a beneficial intestinal environment (Van Heugten et al., 2003), and improving intestinal immunity (Jurgens et al., 1997) have all been suggested as potential modes of action of yeast products. Shen et al. (2009) showed that YC had a positive effect on gut health and immune system. We speculate it may possibility affect by the concentration of YC in diet. Shen et al. (2009) showed that it was increased significantly 5 g/kg YC, but not significantly in a higher YC diet. It means that lower doses YC have a better effect than higher doses. Because the immune response need a higher dosages of YC are required to achieve beneficial effects. The result was similar to Gao et al. (2008). Thus, we need to use a more appropriate dose for the next step to study the mechanism of YC.