Introduction
The overproduction of reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), hydroxyl radical (·OH), and superoxide anion (O2-) are continuously produced from various physiological responses and can cause lipid peroxidation and protein damage that leads to oxidative stress (Cabiscol et al., 2000; Butterfield and Sultana, 2011). Also, nitric oxide (NO) can react with O2- to produce peroxynitrite, relatively more reactive and toxic oxidant, leading to oxidative stress in cells and tissues. Particularly, neurons in the central nervous system of the brain are more vulnerable to oxidative stress than other organs (Chen et al., 2012). The brain has a higher oxygen demand for metabolism than other organs, while it has low concentrations of antioxidants and related enzymes (Lehtinen and Bonni, 2006). Due to the abundance of unsaturated fatty acids in the brain, lipid peroxidation occurs more easily than other organs (Floyd and Carney, 1992). These oxidative stress cause damage to cells and tissues, and if the damage is continuously unresolved, then the cells can activate apoptosis-related pathway (Fulda et al., 2010). This neuronal apoptosis is known to be involved in the preceding and worsening of aging and disease in the human body (Stanely Mainzen Prince and Menon, 2001; Szuster-Ciesielska et al., 2001).
SH-SY5Y human neuroblastoma cells have been widely used as a cellular model for studying neuronal function and neuronal apoptosis, since it shows the characteristics neuron-like behavior in response to neurotoxin. Many previous studies have suggested that SH-SY5Y cells are appropriate for studying neuroprotective role of natural sources in neurodegeneration research such as Alzheimer’s disease or Parkinson’s disease (Cheung et al., 2009; Agholome et al., 2010; Lopes et al., 2010). Therefore, we investigated the effect of Samultang using SH-SY5Y neuronal cells against oxidative stress-mediated neuronal apoptosis.
Samultang is a traditional oriental medicine and also known as Si-wo decoction (China), or Shimotsu-to (Japan). It is a prescription and consists of four herbs; Paeonia lactiflora (PL), Ligusticum striatum (LS), Rehmannia glutinosa (RG), and Angelica gigas (AG) at the ratio of 1 : 1 : 1 : 1. Samultang has been known for prevention from various type of diseases such as blood (Lee et al., 2014), liver (Kim et al., 2003), and heart (Shin, 1996). Recently, many studies have reported the novel pharmacological effects of Samultang such as anti-inflammatory (Choi et al., 2006), anti-stress (Ryu et al., 2001), and anti-cancer (Tagami et al., 2004). Also, Samultang showed memory improvement effects from the scopolamine-induced cognitive deficit in rats (Watanabe et al., 1991). However, anti-oxidant activity and neuro-protective mechanism of Samultang have not been understood yet. Therefore, the aim of the present study was to evaluate the anti-oxidant effect against free radicals and protective activity against oxidative stress of Samultang in H2O2-induced SH-SY5Y neuronal cells.
Materials and methods
Preparation of samples
Samultang and its four ingredients, PL, LS, RG, and AG, used in this research were provided by the Gyeongnam Oriental Anti-aging Institute (Sancheong, Korea). Samultang consists of four herbs; PL, LS, RG, and AG at the ratio of 1 : 1 : 1 : 1. The four herbs were dried by hot air and stored at - 5℃ until extraction. The water extracts of Samultang and its four herbal plants, PL, LS, RG, and AG, were prepared by adding 20 times of purified water followed by heating for 3 h at 90℃. The extracts were filtered by using No. 2 filter paper (Whatman, Kent, UK) and evaporated at 40℃ and stored at 4℃ in a refrigerator.
Instruments and reagents
1,1-Diphenyl-2-picrylhydrazyl (DPPH) and 3-(4,5-dimethylthiazol-2-yl)-2,3-diphenyl tetrazolium bromide (MTT) were obtained from Sigma Chemical Co. (St Louis, USA). Sodium pentacyanonitrosylferrate (III) dihydrate (SNP) and H2O2 were supplied by Junsei Chemical Co. (Tokyo, Japan). Dulbecco’s modified eagle medium (DMEM), penicillin/streptomycin, and fetal bovine serum (FBS) were obtained from Welgene (Daegu, Korea). Dimethyl sulfoxide (DMSO) and dichloro-dihydro-fluorescein diacetate (DCFH-DA) were purchased from Sigma Chemical Co. (St Louis, USA).
DPPH radical scavenging activity
The DPPH scavenging activity of Samultang and its four ingredients, PL, LS, RG, and AG, were estimated according to the method of Hatano et al. (1989). In a microplate, sample and DPPH (60 μM) solution were mixed equally and incubated for 30 min at room temperature. After incubation, the absorbance was measured at 540 nm using a microplate reader.
DPPH radical scavenging activity (%) = [(Ac – As)/Ac] × 100
(Ac: absorbance of the control, As: absorbance of the sample/standard)
·OH scavenging activity
The scavenging activity of ·OH radicals was measured according to the method of Chung et al. (1997). The reaction mixture contained 1400 μL of sample solutions, 200 μL of 10 mM 2-deoxyribose, and 200 μL of 10 mM FeSO4·7H2O2-EDTA were gently mixed and incubated at 37℃ for 4 h. After incubation, adding 2.8% trichloroacetic acid and 1 mL of 0.1% thiobarbituric acid solution. The mixture was boiled for 20 min. The sample solutions cooled in a water bath, and ·OH scavenging activity was measured at 490 nm (Model 680, Bio-Rad, Hercules, USA).
NO radical scavenging activity
The NO scavenging activity of the extracts was estimated according to the method described by Marcocci et al. (1994). Samples of each concentration dissolved in methanol were added to the 10 mM SNP and reacted at room temperature for 150 min. The reaction mixtures were mixed with Griess reagent at 1 : 1 volume ratio in a 96-well plate. Then the samples incubated at room temperature for 30 min, and the wells were read by FLUOstar OPTIMA (BMG Labtech, Ortenberg, Germany) at a wavelength of 540 nm.
Cell culture
SH-SY5Y cells were obtained from KCLB (Korea Cell Line Bank, Seoul, Korea). The cells were cultured at 37℃ in 5% CO2 incubator with DMEM containing 1% penicillin/streptomycin and 10% FBS. Cells were sub-cultured with 0.05% trypsin-EDTA in phosphate buffered saline.
MTT assay
Cell viability was determined by MTT assay (Mosmann, 1983). After cells became confluence, the cells were seeded at 3 × 104 cells/well into a 96-well plate, for 24 h incubation. The cells were treated with various concentrations of Samultang (10, 50, and 100 μg/mL) for 4 h. The cells were stimulated with 300 μM of H2O2 for 24 h. The MTT solution was added to the each well and incubated for 4 h at 37℃. After incubation, medium containing MTT was removed. The intracellular formazan product was dissolved in 200 μL of DMSO and absorbance was measured at 540 nm using a microplate reader.
Measurement of ROS production
The ROS scavenging activity was measured using DCFH-DA (Cathcart et al., 1983). SH-SY5Y cells were seeded at 3 × 104 cells/well into a 96-well plate and incubated for 24 h. The cell was pretreated with various concentrations of Samultang (10, 50, and 100 μg/mL). After incubating for 4 h, the cells were treated with 300 μM of H2O2 for 24 h. The cell was incubated with 80 μM DCFH-DA for 30 min at 37℃. The fluorescence of wells was read by FLUOstar OPTIMA (BMG Labtech, Ortenberg, Germany) at the excitation absorbance of 480 nm and the emission absorbance of 535 nm.
Statistical analysis
Statistical significance was verified by performing one-way ANOVA, followed by Duncan’s multiple range test using the program IBM SPSS version 23 (IBM Corporation, NY, USA). Significance was set at p < 0.05. All values are expressed as mean ± standard deviation (SD).
Results and Discussion
Free radicals and ROS such as O2- and H2O2 are known to be generated in the nervous system and the brain, and constantly be formed in the human body (Aruoma, 1998). Constant exposure and irreversible damage from oxidative stress in neuronal cell lead to neurodegenerative diseases (Klepac et al., 2007; Labbadia and Morimoto, 2013). Thereby, natural herbs or chemicals from food with the beneficial effect against oxidative stress have been reported as therapeutic agent for neurodegenerative disease (Kim et al., 2009). The most common method to screen the anti-oxidant activity is DPPH radical scavenging assay (Wang et al., 2006), which is measuring the degree of discoloration of the original purple color as an index by reducing aromatic compounds and amines due to the electron donating ability of the antioxidant. Here, we investigated the antioxidant activity of Samultang extracts (50, 100, 250, 500, and 1000 μg/mL) under in vitro>. As shown by Table 1, the DPPH radical scavenging capacity of Samultang was increased in a concentration-dependent manner. At the concentration of 100 μg/mL and 1000 μg/mL, Samultang showed the DPPH scavenging activity of 52.22% and 90.31%, respectively.
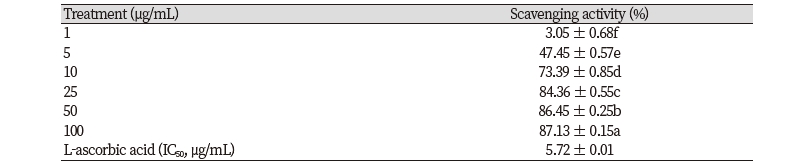
·OH radical is the most reactive ROS and is known to react with biomolecules such as proteins, lipids, sugars, and DNA to induce oxidative damage to the cells and to cause serious diseases in the body (Ashok and Ali, 1999). Excessive ·OH radical generation can damage cell, leading to cell death and apoptosis (Althaus et al., 1994; Ray et al., 2012). The result of ·OH radical scavenging activity of Samultang was shown in Table 2. The treatment of Samultang (1, 5, 10, 25, 50, and 100 μg/mL) increased ·OH radical scavenging activity in a concentration-dependent manner. The strongest ·OH radical scavenging activity was observed in the concentration of 100 μg/mL, showing 87.13% of scavenging effect.
NO is responsible for various types of neurodegenerative disease due to its reactive ability with O2- to form peroxynitire, which is toxic free radical (Sainani et al., 1997). Overproduction of NO can occur DNA damage and cell death (Dawson et al., 1992). Our result revealed that NO radical scavenging capability was increased by treatment with Samultang (5, 10, 25, and 50 μg/mL) as concentration-dependent manner (Table 3). According to previous study, pretreatment of Samultang exhibited potent scavenging activity in DPPH and O2- assay (Um et al., 2017). In addition, Samultang increased superoxide dismutase activity and xanthine oxidase inhibition activity without any toxic effect in mice model (Lee et al., 2010), supporting that Samultang may have the protective activity against free radical-induced damage.
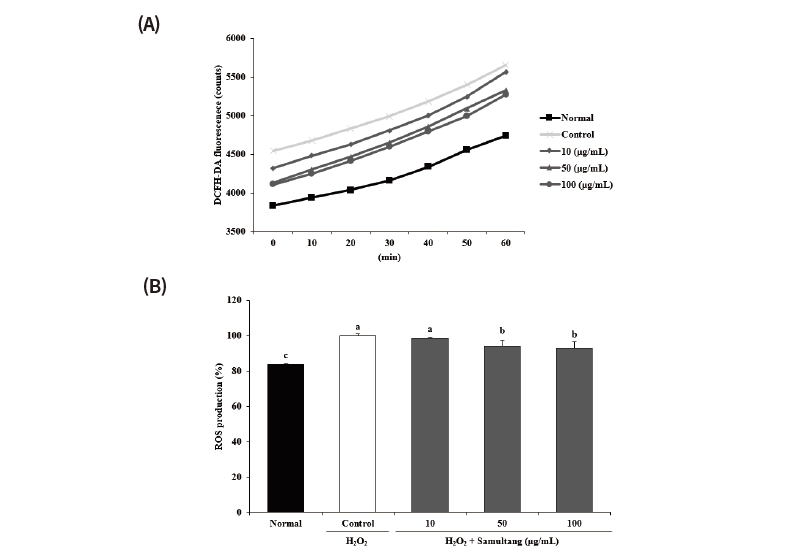
Fig. 2. Effects of Samultang on ROS production in H2O2-treated SH-SY5Y cells. (A) Time-course changes of fluorescence intensity with Samultang. (B) The production of ROS treated with Samultang. Values are mean ± SD. a - c: Means with the different letters are significantly different (p < 0.05) by Duncan's multiple range test. The results were obtained in three independent experiments.
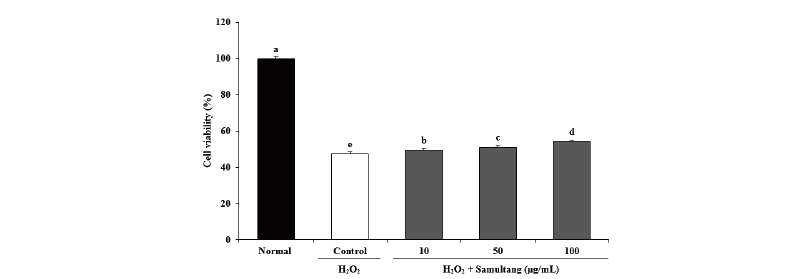
We further studied the protective effects of Samultang in a cellular system using SH-SY5Y cells. This cell is widely used as a model system of neuronal cell death induced by oxidative stress (Fordel et al., 2006). Therefore, the experimental model of oxidative damage in SH-SY5Y cell by free radicals may be useful for discovering effective protection ability from free radicals. To investigate the effects of Samultang on cell viability of H2O2-stressed SH-SY5Y neuronal cells, the cells were pre-treated with various concentration of Samultang (10, 50, and 100 μg/mL) and insulted with 300 μM H2O2. Fig. 1 showed the neuro-protective effects of Samultang on H2O2-induced SH-SY5Y cells under the MTT assay. The treatment of 300 μM H2O2 diminished cell viability to 47.54% compared to 100% of the normal group. However, when cells were treated with Samultang, cell viability was significantly elevated from the H2O2-induced neuronal cell death. In particular, the treatment with 50 μg/mL and 100 μg/mL of Samultang led to the increase of cell viability up to 51.09% and 54.32%, respectively. The present results indicated that the Samultang had protective effects against H2O2-induced oxidative stress in SH-SY5Y cells.
ROS has a high reactivity to escape the supernatant electrons from the destabilized or supersaturated state, thereby causing oxidative stress to the cell lipids, proteins, and nucleic acids. In this study, we investigated the inhibitory effect under SH-SY5Y cells from H2O2-induced oxidative damage. At 60 min, ROS production was higher in the control group than in the normal group. However, the amount of ROS was decreased by treatment of Samultang and the lowest value was found at the concentration of 100 μg/mL, showing 93.19% (Fig. 2). These findings demonstrated that Samultang may attenuate oxidative stress by inhibiting intracellular ROS levels, suggesting its anti-oxidant effect in H2O2-induced SH-SY5Y cells. It was reported that Samultang significantly attenuated neuronal apoptosis against oxidative stress by regulation of apoptotic signaling pathway in SK-N-MC cell and HT22 hippocampal cell line (Kang et al., 2000; Lee and Kim, 2009). Additionally, Samultang suppressed apoptosis via caspase inhibition in glial cell, suggesting that Samultang may responsible for protection of brain damage (Kim et al., 2000).
Based on these results, we evaluated comparative study on antioxidant activity of four herbal plants in Samultang by using DPPH and ·OH radical scavenging activity. As shown in Table 4, the DPPH radical scavenging capacity was increased in all groups. The treatment of 100 μg/mL of PL, AG, RG, and LS showed 53.31%, 14.29%, 35.04%, and 13.56% of DPPH radical scavenging activity, respectively. Especially, at the concentration of 100 μg/mL of PL showed the highest DPPH scavenging activity. The result of ·OH radical scavenging activity are shown in Table 5. The strongest ·OH radical scavenging activity was found in PL. The treatment of 100 μg/mL of PL showed 77.10% of ·OH radical scavenging activity. Among the four kinds of herbs, we confirmed that PL showed the strongest DPPH and ·OH scavenging activity at the low concentration. In agreement with our study, the highest levels of antioxidant activity were observed in the PL treatment group, which had the highest polyphenol content among the Samultang ingredients (Choi et al., 2008). PL has been reported to have antioxidant properties and protective effect against oxidative stress. According to Heo et al. (2013), treatment of PL inhibited ROS production by transcriptional activation of manganese superoxide dismutase and catalase. In particular, PL treatment protected dopaminergic SH-SHSY neuronal cell death via induction of heme oxygenase 1 gene expression, suggesting that protective effect of PL on neuronal cell is likely medicated by regulation of anti-oxidant signaling pathway (Lee et al., 2016). Although further studies are needed to investigate the protective effect and molecular mechanisms of PL and its active compounds, these evidences supported that Samultang plays a crucial role in protection on neuronal cell death against oxidative stress.
Conclusion
Samultang significantly increased in DPPH, ·OH, and NO radical scavenging activity. Furthermore, Samultang showed the effect of increase in the cell viability and decrease in the ROS production in the H2O2-induced SH-SY5Y cells. In addition, its four herbs, PL, LS, RG, and AG, exerted scavenging activities of DPPH, ·OH, and NO radicals. Especially, PL showed the highest anti-oxidative effect among other herbal plants of Samultang. Taken together, our studies suggest that PL and Samultang are responsible for the protection against the oxidative stress from the H2O2-induced neuronal cell death.