Introduction
The eggshell quality is important for both producers and consumers. Among various measurements of egg quality, eggshell strength and intensity of brown eggshell color are the greatest interests because of their direct impact on egg productivity and safety as well as consumers’ preferences (Odabasi et al., 2007; Samiullah et al., 2015; Sirri et al., 2018).
In general, the eggshell strength and intensity of brown eggshell color in brown-egg laying hens gradually decrease with hens’ age because of the fact that egg size increases with age but the aged hens have limited ability to support appropriate eggshell formations (Odabasi et al., 2007; Samiullah et al., 2016; Hy-Line International, 2018). Thus, poultry researchers have made a lot of efforts to improve eggshell strength and intensity of brown eggshell color in the aged laying hens with various methods such as modifications in dietary compositions and raising systems (Roberts, 2004; Oh et al., 2018). Especially, different sources and concentrations of diverse macro or trace minerals in diets have been widely studied to improve eggshell strength and intensity of brown eggshell color because the main components of eggshells are minerals (more than 95%; Leeson and Summers, 2008). Increasing Ca concentrations and a larger particle size of Ca sources in diets have been shown to improve the eggshell strength of aged hens (Safaa et al., 2008; An et al., 2016). Different ratio of Ca to P in diets has also been tried to improve the eggshell strength (Lim et al., 2003). In our previous experiment, increasing Mg concentrations in diets improved the eggshell strength of aged laying hens (Kim et al., 2013); however, we observed decreased intensity of brown eggshell color by feeding diets containing high amounts of Mg due to increasing accumulation of Mg and its white element color. Although there are limited researches regarding the effect of dietary treatment on intensity of brown eggshell color, some experiments showed the positive or negative effect of dietary factors on intensity of brown eggshell color in laying hens. Seo et al. (2010) reported that feeding diets containing high amounts of Fe improved intensity of brown eggshell color (i.e., eggshell redness) in brown egg-laying hens. In contrast, it is reported that dietary vanadium decreased intensity of brown eggshell color but dietary supplementation of vitamin C ameliorated discoloration of eggshells (Odabasi et al., 2006). However, limited information regarding the effects of dietary components on eggshell strength and intensity of brown eggshell color is still available in the aged laying hens. In addition, data regarding nutrient utilization of aged laying hens with different eggshell strengths or different intensities of brown eggshell color are scarce, which may be the reason why poultry nutritionists can hardly find the promising solutions to improve the eggshell quality of aged laying hens.
The objective of the present experiment, therefore, was to compare nutrient utilization in diets for aged laying hens with different eggshell strengths or different intensities of brown eggshell color.
Materials and Methods
Animal selection
All experimental procedures were reviewed and approved by the Institutional Animal Care and the Use Committee at Chung-Ang University. A total of 500 92-wk-old Hy-Line Brown laying hens were initially used to investigate the intensity of brown eggshell color. The intensity of brown eggshell color was determined using the eggshell color fan (Samyangsa, Korea) with 15 scales (15 = dark brown and 1 = light white). The intensity of brown eggshell color from all eggs of individual hens was measured daily during the first 10 days, and then, a total of 100 hens with dark brown eggshell (DBE) and 100 hens with light brown eggshell (LBE) were chosen. All eggs from those hens were measured for eggshell strength with the texture analyzer (model TAHDi 500, Stable Micro System, Godalming, UK) and intensity of brown eggshell color with the Minolta Chroma Meter CR-400 (Minolta, Osaka, Japan) during additional 7 days. Based on eggshell strength and eggshell color of lightness (L*), redness (a*), and yellowness (b*), 20 hens with a strong eggshell strength (SES) and 20 hens with a weak eggshell strength (WES) were chosen. Likewise, 20 hens with DBE and 20 hens with LBE were chosen again. The remaining 120 hens were discarded from the experiment. Finally, all eggs from 80 hens were also reexamined for additional 6 days to select 12 representative hens per each eggshell quality (i.e., 12 hens for SES, WES, DBE, or LBE). All hens were raised according to the guidelines for Hy-Line Brown laying hens (Hy-Line International, 2018).
Metabolism trial
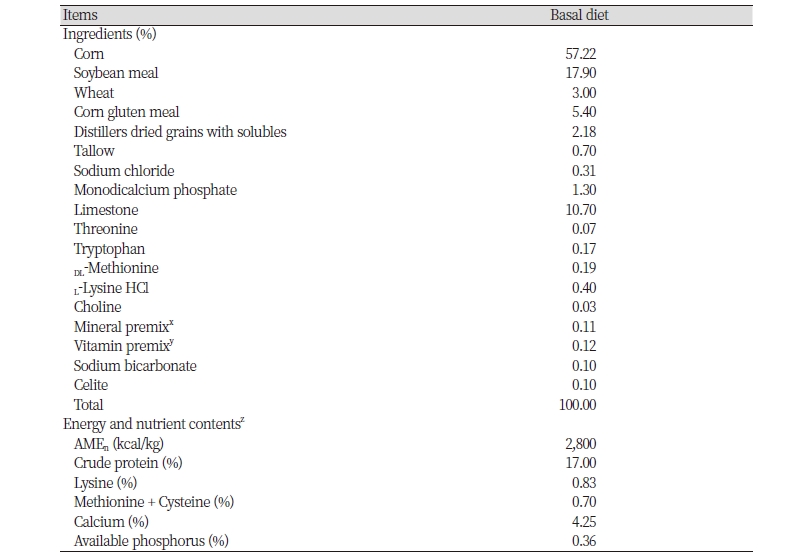
Two separate experiments were performed to compare nutrient utilization in aged laying hens with different eggshell strengths (SES vs. WES) or different intensities of brown eggshell color (DBE vs. LBE). All hens were placed in 48 battery cages with 1 bird per cage. During the entire experiment, all hens were fed a common commercial layer diet that satisfied energy and nutrient recommendations for aged laying hens (Hy-Line International, 2018; Table 1). Chromic oxide was included at the level of 0.5% in the diet as an indigestible marker to determine the apparent total tract retention (ATTR) of energy and nutrients in diets (Gutierrez del Alamo et al., 2008; Choi et al., 2019).
All hens were adapted to the diet for 10 days and the fresh excreta were continuously collected for additional 5 days. Excreta samples were dried in a forced-air drying oven at 60℃ for 48 hours and ground prior to chemical analyses.
The gross energy (GE) concentrations in the diet and excreta were determined using a bomb calorimetry (Model 6400, Parr instruments Co., Moline, USA). The diet and excreta were also analyzed for dry matter (DM, method 930.15; AOAC, 2007), nitrogen (N, method 990.03; AOAC, 2007), acid-hydrolyzed ether extract (AEE, method 996.01; AOAC, 2007), neutral detergent fiber (NDF, method 973.18), acid detergent fiber (ADF, method 973.18), and ash (method 942.05; AOAC, 2007). The diet and excreta were also analyzed for Ca, P, Cu, Fe, Zn, and Mn using an inductively coupled plasma spectrometer (Optima 5300 DV, Perkin Elmer Inc., Shelton, USA) according to the method described by Edwards and Gillis (1959). The values for the ATTR of GE and nutrients in diets for aged laying hens were calculated from the general equations in indicator methods (Hill and Anderson, 1958).
Statistical analysis
All data were analyzed by ANOVA as a completely randomized design using the PROC MIXED procedure of SAS (SAS Institute, Cary, USA). Each hen was considered an experimental unit (n = 12) in all analyses. Outlier data were checked using the UNIVARIATE procedure of SAS (Steel et al., 1997), but no outlier was identified. Treatment was included as a fixed effect in the model and the LSMEANS procedure was used to calculate treatment means (Seo et al., 2018). Significance for statistical tests was set at p < 0.05.
Results
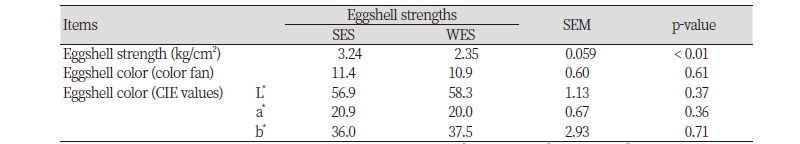
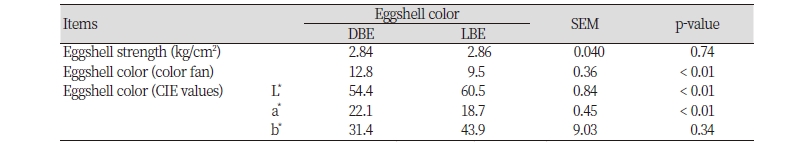
As we intended, laying hens with SES had a greater (p < 0.01) eggshell strength than those with WES; however, no differences in intensity of brown eggshell color were observed in laying hens with different eggshell strengths (Table 2). Likewise, laying hens with DBE had a darker (p < 0.01) intensity of brown eggshell color (eggshell color fan score and CIE values for L* and a*) than those with LBE with no differences in eggshell strength (Table 3).
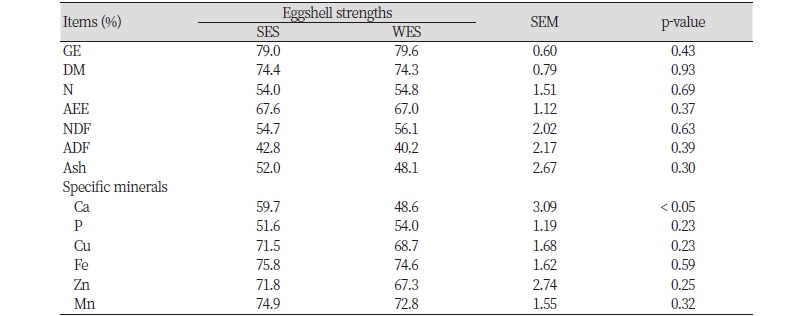
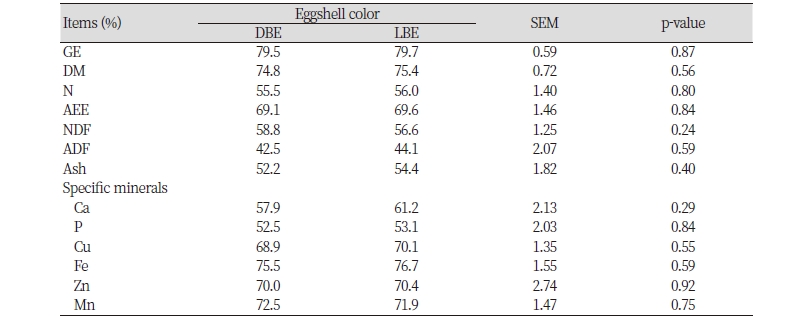
In energy and nutrients utilization in diets, aged laying hens with SES (59.7%) had a greater (p < 0.05) ATTR of Ca (p < 0.05) than those with WES (48.6%; Table 4). However, the ATTR of GE and other nutrients (i.e., DM, N, AEE, NDF, ADF, ash, P, Cu, Fe, Zn, and Mn) was not different between the aged laying hens with different eggshell strengths (SES vs. WES). However, no differences in the ATTR of GE and all nutrients were observed in the aged laying hens with different intensities of brown eggshell color (DBE vs. LBE; Table 5).
Discussion
It is well known that eggshell strength of laying hens gradually decreases as hens become aged because egg size and weight increases with age but the aged hens have the limitation to support these physiological changes by compensating the eggshell size and thickness (Odabasi et al., 2007; Samiullah et al., 2016; Hy-Line International, 2018). As Ca is the primary mineral in eggshells, therefore, many researchers have tried and shown to improve the eggshell strength in the aged laying hens by feeding diets containing coarsely ground form of Ca sources and high Ca concentrations (Safaa et al., 2008; An et al., 2016), which is likely validated by our observation for a less Ca utilization for aged laying hens with WES than those with SES. It should be noted, however, that one flock of aged laying hens consists of both hens with SES or WES and each group has different abilities to utilize dietary Ca. Thus, it may be ideal to use different sources and concentrations of dietary Ca for aged laying hens with different eggshell strengths because feeding diets containing very high concentrations of Ca may have negative effects (e.g., imbalance of Ca and P) on aged laying hens with SES. However, it is questionable to adopt our suggestion in the practical layer production. However, our result for the relationship between Ca utilization and eggshell strength in the aged laying hens may be used to develop new layer breeds with a high eggshell strength.
Previous experiments indicated that additional supplementation of some traces minerals such as Zn, Cu, and Mn in diets had benefits on eggshell strength of aged laying hens (Lim and Paik, 2003; Mabe et al., 2003; Stefanello et al., 2013). However, we failed to find any differences in other mineral utilization between the aged laying hens with different eggshell strengths. Thus, the beneficial effects reported previously may not be explained by different utilization in those minerals.
Consumers in different countries have different preferences on the egg color (i.e., eggshell colors) with most consumers in Asia, Australia, and South America showing a strong preference on brown eggs (Odabasi et al., 2007). Although intensity of brown coloration in eggshell is not directly related to egg quality such as nutrient composition and shell strength in eggs, consumers prefer dark brown eggs to light brown eggs because of misconceptions that light brown eggs may be produced by hens with health problems and high stress. Therefore, increasing production of light brown eggs may lead to a decrease in their choice of consumers, which results in economic loss for egg producers.
Several factors have been reported to affect the intensity of eggshell color in brown eggs (Roberts, 2004; Odabasi et al., 2007; Samiullah et al., 2015). Age is one of important factors because the aged laying hens generally produce lighter and faded eggs because of insufficient ability to synthesize eggshell colorants (i.e., protoporphyrin IX) to cover increased egg size with age (Odabasi et al., 2007; Samiullah et al., 2015). However, very few researches have been performed to study the effect of dietary treatments on brown coloration in eggs, possibly due to the undefined notion that the intensity of brown eggshell color is determined primarily by genetics, diseases, and environmental stress (Chousalkar and Roberts, 2009; Samiullah et al., 2015, 2016).
The intensity of brown eggshell color is affected by the synthesis of protoporphyrin IX and its transfer to eggshells in the uterus (Samiullah et al., 2015). It has been reported that some trace minerals such as Fe, Cu, Mn, and Zn can function as a chelating agent, and therefore, they may play a role in the metabolism of porphyrin molecules (Solomon, 1987). It is likely, therefore, that increasing utilization of Cu, Fe, Zn, and Mn in diets may have a benefit on synthesis of protoporphyrin IX and its transfer to eggshells, which subsequently increases intensity of brown color in eggshells. However, our results indicated that no differences in the ATTR of Cu, Fe, Zn, and Mn of aged laying hens with different intensities of brown eggshell color. Other nutrient utilizations were also not different of aged laying hens with different intensities of brown eggshell color. These results may indicate that the intensity of brown eggshell color is independent of energy and nutrient utilization in diets for aged laying hens. However, there have been some experiments reporting the improvement in intensity of brown eggshell color by dietary treatments such as δ-aminolevulinic acid, vitamin C, and Fe (Odabasi et al., 2006; Seo et al., 2010; Wang et al., 2011). Therefore, further researches are required to find the effective dietary means to improve intensity of brown eggshell color in the aged laying hens.
Conclusion
Aged laying hens with SES have a greater ability to utilize dietary Ca than those with WES, despite no differences in energy and other nutrient utilization. However, it is likely that energy and nutrient utilization in diets is not different between the aged laying hens with different intensities of brown eggshell color.
Acknowledgement
This research was carried out with the support of the Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ01256701), Rural Development Administration, Republic of Korea.
Authors Information
Jong Hyuk Kim, Animal Science and Technology, Chung-Ang University, Postdoctoral researcher, https://orcid.org/0000-0003-0289-2949
Gi Ppeum Han, Animal Science and Technology, Chung-Ang University, Ph.D. student, https://orcid.org/0000-0001-7794-2213
Hwan Ku Kang, National Institute of Animal Science, Rural Development Administration, Researcher, https://orcid.org/0000-0002-4286-3141
Dong Yong Kil, Animal Science and Technology, Chung-Ang University, Professor, https://orcid.org/0000-0002-9297-849X