Introduction
Soybean meal (SBM) is the important component of animal feed Soybean is the most acknowledged source of plant protein, which also contributes to a wide range of health benefits (Sanjukta and Rai, 2016). SBM also contains anti-nutritional factors (ANF) and antigenic proteins (Upadhaya et al., 2016). Which includes trypsin inhibitors (TI), flatulence-producing compounds, phytic acids, the agglutinins. These ANF obstruct the absorption and utilization of nutrients in animal husbandry (Oomah et al., 2011; Elinge et al., 2012). To overcome the negative effects of ANF, microbial fermentation of SBM could play an influential role to remove ANF and allergenic properties of SBM (Hong et al., 2004; Frias et al., 2007). Bacillus amyloliquefacienss one of the microbial that has shown significant effect in improving the nutritional quality and bioactivity by removing the protein and carbohydrate-based anti-nutritional factors (ANF), as well as allergens. The protein content in SBM increased by 6.42% after fermentation with B. amyloliquefaciens (Chi and Cho, 2016). Compared with the unfermented SBM, fermented soybean meal (FSBM) has been introduced as a novel protein source with reduced anti-nutritional and allergenic properties (Roh et al., 2015), which is beneficial for the synthesis of amino acids and tissues in the animal body, especially to young animals improving the immune ability of the body and resisting invasion by pathogens (Yang et al., 2014). FSBM as a replacement for conventional SBM improved the efficiency of nutrient utilization, intestinal micro-ecology (Feng et al., 2007) and diarrhea score in nursery pigs, suggesting that FSBM provides beneficial effects on growth and nutrient utilization (Kim et al., 2010).
Studies on the efficacy of SBM fermented by Bacillus amyloliquifaciens on weaning pig performance, nutrient digestibility, blood profiles are limited. Therefore, more studies are needed to compare the effects of fermented by the said bacteria and unfermented SBM in weaning pigs. Thus, he objective of the experiment was to evaluate the effect of dietary supplementation of Bacllus amyloliquifaciens fermented SBM on the growth performance, nutrient digestion, blood profile and fecal microflora of FSBM on weaning pigs.
Materials and methods
Fermented soybean meal (FSBM: Soytide)
This study used fermented soybean meal (FSBM: Soytide) obtained from CJ Global Food and Bio Company. The soytide preparation was as follows: B. amyloliquefaciens, isolated from the Korean traditional fermented soybean, was cultured in Nutrient Broth (Becton, Dickinson and Company, Franklin Lakes, USA) for 24 h at 37℃ with shaking. The cell was adjusted to 109 CFU/mL with sterilized physiological saline and used as inoculums for solid-state fermentation. 30 kg of soybean meal was mixed with 20 L of distilled water, and the mixture was heated to 100℃ for 30 min in a commercial autoclave. Then, they were cooled to room temperature inoculated with 3 L of B. amyloliquefaciens and incubated for 24 h at 37℃ and 95% humidity in a constant temperature humidity chamber. After solid-fermentation, samples were air dried at 60℃ for 8 h and stored at 4℃.
Experimental design, animals, diets and facilities
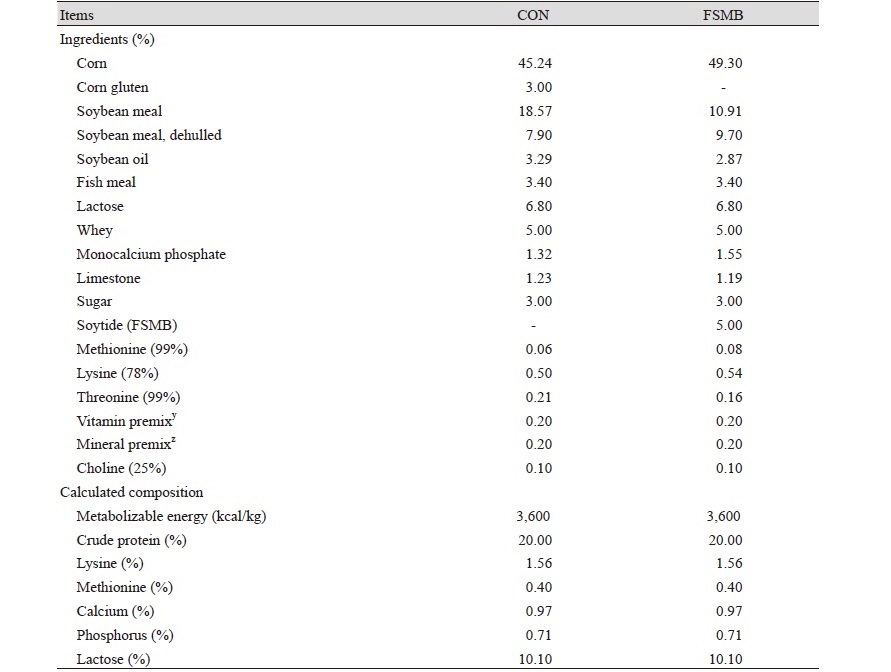
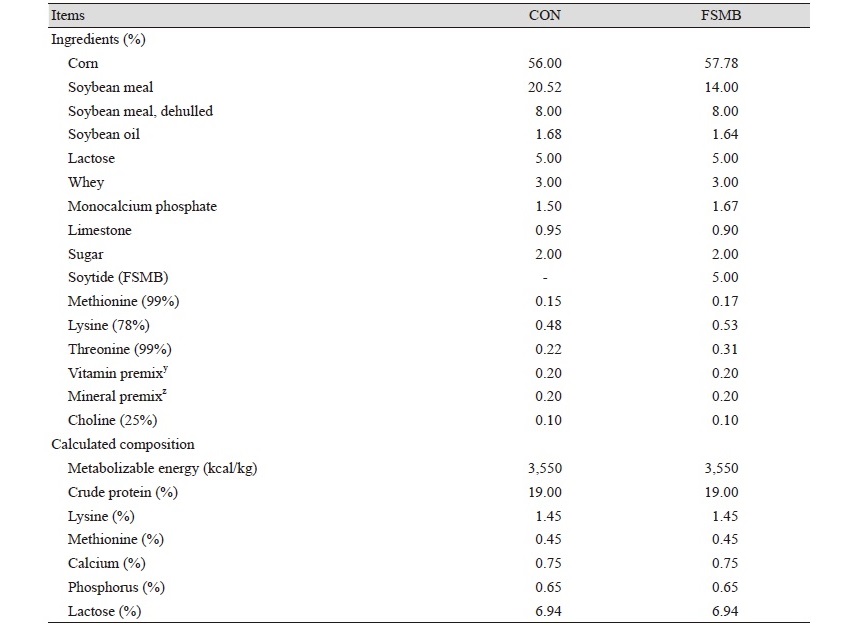
The total animals were 100 (2 treatments) weaned pigs ([Landrace × Yorkshire] × Duroc), initial body weight (BW) 8.27 ± 1.10 kg, all weaned pigs randomly allotted to 2 treatments based on initial BW. Each treatment had 10 replicate pens and each pen had 5 pigs. The dietary treatments included: 1) control, basal diet (CON); 2) Basal diet + 5% soytide (FSMB). The diets were formulated based on NRC (2012) recommendations. The environmental temperature was maintained at 26℃ for the first week of the experiment. The environmental temperature was reduced by 1℃ per 2 weeks over the next 6 weeks. The relative humidity was 60 - 65%. And the water was controlled so that it could be eaten freely using an automated food processor. Water pressure was controlled at 0.475 L/min. Providing 16 hours of light per day, and these weaning pigs were provided light for 16 h daily throughout the experiment. They were fed for 6 weeks.
Experimental sample and measurements
The experiment was divided into 2 phases. The individual body weight and feed consumption on pen basis were recorded during, the first week, the third week and the sixth week. The average daily gain (ADG), average daily feed intake (ADFI) and gain to feed ratio (G : F) was calculated from these data. The nutrient digestive rate was calculated by adding 0.2% of chromium (Cr2O3) as an indicator at the end of the week (6th week) and then sampling the feces after being fed for 7 days using the anus massage method. The collected feces were dried at 60℃ for 72 hours, then crushed with a mill to be used for analysis. Cr2O3 mix of the general ingredients and the display of the feed and analyzed according to the method in AOAC (2000). Three pigs were selected randomly from each pen and bled via jugular venipuncture at the end of the experiment (week 6). Blood samples were collected into 5 mL vacuum tubes with K3EDTA (Becton, Dickinson and Company, Franklin Lakes, USA). Then the blood was extracted and separated for 15 minutes with a separation of 3,000 and placed on ice until analysis (Wang et al., 2019). For fecal microorganism, the fecal samples were collected using the anus massage from 2 pigs randomly selected from each pen (1 gilt and 1 barrow) at the end of the six weeks. Then these fecal samples were transported to the laboratory as soon as possible, where analysis was immediately carried out (Li et al., 2019). The composite fecal sample (1 g) from each pen was diluted with 9 mL of 1% peptone broth (Becton, Dickinson and Company, Franklin Lakes, USA) and homogenized. Viable counts of bacteria in the fecal samples were then conducted by plating serial 10-fold dilutions (in 1% peptone solution) onto MacConkey agar plates (Difco Laboratories, Detroit, USA) and Lactobacilli medium III agar plates (Medium 638, DSMZ, Braunschweig, Germany) to isolate the Escherichia coli and Lactobacilli, respectively. The MacConkey agar plates were then incubated for 24 h at 37℃, and the Lactobacilli medium III agar plates were incubated for 48 h at 39℃ under anaerobic conditions. The E. coli and Lactobacilli colonies were counted immediately after removal from the incubator.
Statistical analysis
All data obtained in the current study were analyzed in accordance with a completely randomized block design using the GLM procedure of SAS (SAS Institute,1996). Duncan's multiple range test was used to compare the means of the treatments. Variability in the data is expressed as standard error of the mean (SEM) A p-value less than 0.05 was considered error (SE) and probability level of p < 0.05 was considered to be statistically significant.
Results
Growth performance
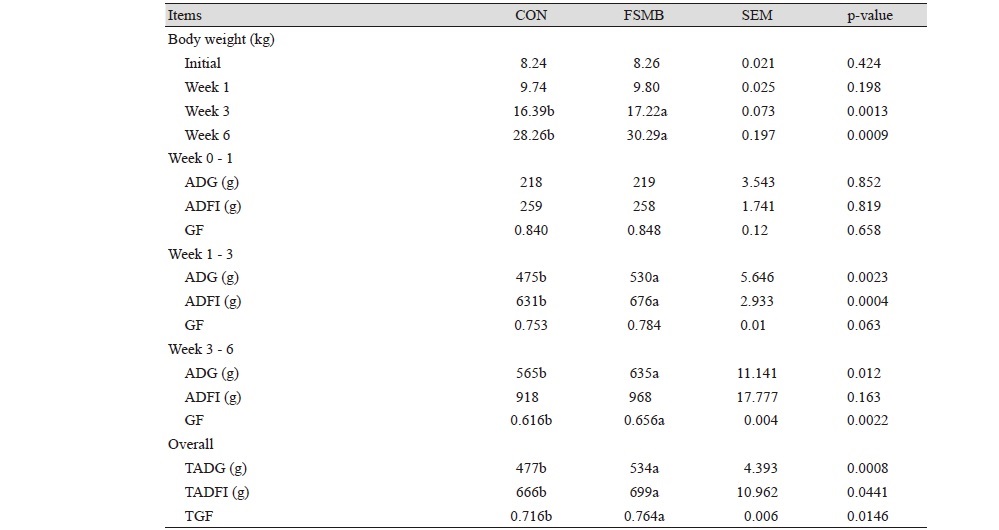
No piglets were found dead because of disease or other reasons throughout the experiment. From 1 to 3 weeks and from 3 to 6 weeks, the average intake and daily weight gain of the experimental group were significantly higher (p < 0.05) than the control group. The gain to feed ratio (G : F) increased in the FSMB group, and there was significant difference (p < 0.05). Compared with the control group, the addition of 5% fermented soybean meal into the feed of the experimental group had a significant effect (p < 0.05) on growth performance parameters during the overall experimental period (Table 3).
Apparent nutrient digestibility
The pigs fed diet with FSBM had no significant effect on the total tract digestibility of dry matter, energy and nitrogen as compared with control (Table 4).
Table 4. Effect of dietary supplementation with soytide on nutrient digestibility in weaning pigs.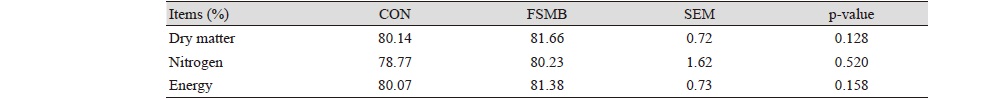
|
|
|
CON, basal diet; FSMB, basal diet + 5% soytide; SEM, standard error of the mean. |
|
Blood profile
The concentrations of blood urea nitrogen (BUN), creatinine and glucose remained unaffected with the supplementation of FSBM in the diet as compared with control diet (Table 5).
Table 5. Effect of dietary supplementation with soytide on blood profile in weaning pigs.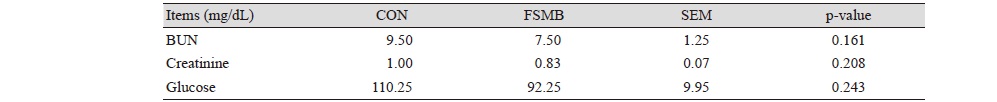
|
|
|
CON, basal diet; FMSB, basal diet + 5% soytide; SEM, standard error of means; BUN, blood urea nitrogen. |
|
Fecal microflora
The supplementation of FSBM did not have any significant effect on the fecal microflora as compared with the control diet (Table 6).
Discussion
Weaning remains a critical phase in pig production and associated with digestive disorders causing reduced growth and diarrhea that are often associated the intestinal villi and micro-ecology of the intestine are relatively vulnerable (Kiers et al., 2003). The results showed that the addition of fermented soybean meal in the diet, significantly improved the growth of weaning pigs, increased the ADG, ADFI, and gain to feed ratio (G : F) compared with the control group. So, it is suggesting that the growth performance of weaned pigs could be improved with the addition of fermented soybean meal in the feed. It is consistent with the results of Song et al. (2010). Cera et al. (1988) reported that the piglets after weaning suddenly change from liquid feed to solid feed, under the action of dry matter, the intestines the villi become shorter and the crypts deepen. This change will last for 7 - 14 days, which seriously affects the secretion and absorption capacity of the piglet's digestive process. Hampson’s studies also found the same results (Hampson, 1986). Incorporation of anti-nutritional factors (ANF) such as glycinin or β-conglycinin to the diet led to reduced weight gain and feed efficiency as well as an increased incidence of diarrhea in pigs (Zhao et al., 2008). These results agree with previous reports that used SBM fermented by Aspergillus oryzae (Kim et al., 2007; Liu et al., 2007) or lactic acid bacteria (Cho et al., 2007; Wang et al., 2007). It can be shown from these studies that the addition of FMSB in the basic diet can significantly enhance the growth performance of weaned pigs, which is consistent with the results of this experiment.
The digestibility of dry matter (DM), energy, crude protein (CP) and ash in FSBM treatment tended to show linear effect (Kim et al., 2007). Min et al. (2004) reported that digestibility of DM and N were higher for pigs fed FSBM diets than pigs fed control diet and the authors suggested that FSBM was absorbed by the pig's gastrointestinal tract, which was beneficial to improve the body's digestion and utilization of feed. Yuan et al. (2017), Feng et al. (2007) and Rojas and Stein (2013) supplementation of FMSB in piglets daily had effect to digestibility of nutrients. However, in the present study our results showed that the digestibility of nutrients (DM, N, and energy) was not affected significantly in weaned pigs after supplementation 5% fermented soybean meal. The possible reason was the inactive microorganisms in fermented soybean meal that aid digestion in the gut. Likewise, Min (2009) reported that FSBM did not affect DM digestibility. But N digestibility was higher as FSBM levels were increased. Also, there were no significant differences in DM and N digestibility between the adding of FSBM in simple diet and non adding FSBM in the complex diet (Min, 2009). There was no significant difference in digestibility when soybean meal fermented by bacillus subtilis was supplemented to the diet of weaning piglets (Hossain et al., 2016). Thus, there is inconsistent in the report regarding the digestibility of nutrients with the inclusion of FSBM in the diet. Inconsistencies in the results of different studies may be related to animal age, diet, management, and environmental factors.
In our study, blood urea nitrogen, creatinine and glucose were not significantly in this experiment. The decrease of blood urea nitrogen can increase nitrogen deposition and increase protein synthesis. BUN is the main end product of protein metabolism in animals. The amount of urea in body depends on the intake of protein in diet, protein catabolism in tissues and liver function. The level of blood urea nitrogen can reflect the metabolism of animal protein and can be used as an indicator of nitrogen utilization and protein deposition (Yang et al., 2014). Creatinine is a product of muscle metabolism, and like urea, is excreted by the kidneys (Baum et al., 1975). Cho et al. (2007) research indicated that fermented soybean meal in diet could reduce BUN level. These results showed that BUN concentrations were affected by FSBM diet. But Yang et al. (2014) reported that there was no obvious correlation with the amount of fermented soybean meal. Although have no significant in blood profiles between FMSB group and CON, but supplementation of FMSB has improvement trend. The insignificance of the results in this study may be related to the sub-health of weaned pigs, with the environment factor and ill management resulting in insignificance.
After the soybean meal is fermented, the degradation of macromolecular protein in fermented soybean meal is beneficial to the digestion and absorption of feed protein by weaned piglets. In addition, the number of probiotics are greatly increased. After entering the intestinal tract of piglets, these beneficial bacteria can stimulate intestinal mucosal immunity. The main intestinal floras in tract of piglets are gram-positive bacteria lactic acid bacteria and gram-negative bacteria E. coli. The Lactobacillus can improve intestinal function, promote nutrient digestion and absorption, and regulate immune function (Vanbelle et al., 1990). Lactic acid bacteria is the main beneficial bacteria in the tract. These lactic acids in the tract can regulate the tract pH, and it is beneficial to growth of beneficial bacteria (Mathew et al., 1998). E. coli is harmful bacteria in the intestinal tract, and it is the most common cause of post-weaning mortality on many farms killing 15 - 20% of the piglets weaned (Hampson, 1986). In the present study the fecal Lactobacillus counts in pigs fed FSBM were not affected significantly compared with those fed control diets. However, there is a tendency in the reduction of E. coli counts, supplement FSBM in pigs diet indicating that FSBM supplementation conferred some positive health effects in weaned pigs by reducing pathogenic microflora in the gastrointestinal tract. There is no significant difference in fecal microorganisms, which may be related to the relative stability of intestinal microorganisms in the experimental animal group.
Conclusion
Dietary supplementation with 5% fermented soybean meal had significant effect on the ADG, ADFI, and gain to feed ratio (G : F) of weaning pigs, but no effects were observed on total tract nutrient digestibility, blood profile as well as fecal microflora. Therefore, more studies are needed with more dose of fermented soybean meal to assess their effect as a functional product in weaning pigs.