Introduction
Buffaloes are known to be more efficient in utilizing fiber component of coarse feed than cattle and they thrive well on crop residues, cropping native varieties, and agri-by products (Punia and Singh, 2001). Mycotoxin contaminations are serious issues in farming and animal husbandry. So far, various mycotoxins have been identified and over 25% of the world annual grain production is contaminated with mycotoxin (Smith et al., 2016). The optimum temperature for growth and production of aflatoxin by Aspergillus parasiticus and Aspergillus flavus is 25 to 35℃ and 28 to 30℃, respectively (Bhat et al., 2010), as highly mutagenic and carcinogenic to animals. As that optimum temperature is provided in warm-temperature zones, aflatoxins are a far greater problem in tropical regions than in temperate zones of the world. However, the movement of agricultural commodities around the globe has made almost every part of the world a target, and no region of the world is free from aflatoxins. Effects of aflatoxins in ruminants are dependent upon multiple factors that include: duration of exposure, type of diet (ruminant diets are quite complex and involve the use of different types of ingredient), status of the animal (age, sex, breed, dry matter intake, general health, immune status, nutritional strategies), and environmental parameters (farm management, hygiene and biosecurity). Aflatoxin B1 is a carcinogen for both animals and humans (Jiang et al., 2012; Mojtahedi et al., 2013; Gallo et al., 2015) and is excreted in milk in the form of aflatoxin M1 (AFM1). When dairy animals consume aflatoxin-contaminated feeds, they metabolically bio transform aflatoxin B1 (AFB1) into a hydroxylated form called AFM1. The aflatoxin metabolite AFM1, is carried over into the milk from around 1 to 6% of the aflatoxin consumed (Pettersson, 2004). In addition, AFB1 contaminated feed showed a reduction in gas production, ammonia and volatile fatty acids (VFA) concentrations, together with an increased bioavailability of AFB1 in rumen fluid (Morgavi and Riley, 2007; Fink-Gremmels, 2008; Pulina et al., 2014). Aflatoxins are highly immunosuppressive as well and effects on animals can be significant, even at low doses (Fink-Gremmels, 2008; Pulina et al., 2014; Gallo et al., 2015). The AFB1 is not only carcinogenic but also teratogenic, hepatotoxic, mutagenic, and immunosuppressive, and the main target organ is the liver (Eaton and Gallagher, 1994). Based on human epidemiological studies, AFB1 is one of the most potent hepatocarcinogen among naturally occurring toxicants and known to cause increased hepatocellular carcinoma in populations exposed to AFB1 via contaminated foods (Groopman et al., 1988). Hence, AFB1 is classified by the International Agency for Research on Cancer (IARC) in Group 1, as carcinogenic to humans, whereas AFM1 is listed in Group 2B, as possibly carcinogenic to humans (IARC, 1993). The objective of the present investigation was to study the impact of different concentration of AFB1 on In vitro rumen fermentation in buffalo diet.
Materials and Methods
Production and determination of aflatoxin
Aflatoxin was produced using the fungal strain A. flavus NRRL 6513 (U.S Department of Agriculture, Illinois, USA) culture was used as inoculant and produced on liquid medium according to method described by Singh and Shamsudeen (2008). To get the fresh spores the culture was regularly sub-cultured on potato dextrose agar (PDA) medium slants and stored at 5℃. Aflatoxin contents were finally quantified using UV-Visible Spectrophotometry (model no: 117, Systronics, Kolkatta, India). Plates were inspected for aflatoxin B1 in a chromate-viewer to find the components by fluorescence. Quantification of AFB1 was determined by visual comparison of fluorescence zone with the known quantity of zone formed by the standards of aflatoxin. AFB1 was separated by scratching the chromatogram.
Experimental design and substrate
Feed sample (wheat straw) was ground to pass a 1 mm sieve and used for experimentation. The study categorized to one of four dietary treatments as basal diet included with 0 (T1), 100 (T2), 200 (T3), 300 (T4) ppb AFB1 for further analysis. The wheat straw was analyzed for dry matter (DM), organic matter (OM), ash, crude protein (CP) and ether extract (EE), while neutral detergent fiber (NDF) and acid detergent fiber (ADF) were also determined as described by Van Soest et al. (1991) and mentioned in Table 1.
Collection of rumen liquor
Fistulated male buffalo, fitted with permanent rumen cannula, about 2 years-old having 250 kg body weight was used as donor animal for collection of rumen liquor. The animal was fed a basal diet of wheat straw offered and water was provided ad libitum and a standard concentrate mixture containing 20% CP and 70% TDN to meet the nutrient requirement for maintenance (AFRC, 1991). Animals were cared for according to the Council of Animal Care guidelines. Approximately 300 mL of rumen liquor was collected from different depths and directions of reticule rumen and transferred into pre heated thermos flask, strained through a fourfold muslin cloth and flushed with CO2. Rumen liquor was collected in the morning before feeding and watering of the animal as per standard procedure. Rumen fluid–medium mixture was prepared under continuous flushing with CO2 to maintain anaerobic condition.
In vitro incubation of substrate and gas production
A total of 200 mg dry weight of each feed substrate was weighed into 100 mL calibrated syringes and incubated with 30 mL of mixed rumen inoculum at 39℃ for 24 h with parallel incubation of blanks (Menke et al., 1979; Menke and Steingass, 1988). Each substrate was incubated in triplicate. The syringes were regularly shaken by hand during the incubation period for proper mixing of feeds with rumen inoculum. After 24 h of incubation period, the gas production was recorded by the displacement of piston during incubation period for test substrate and blank syringes. The net gas produced due to fermentation of substrate was calculated by subtracting the value of gas produced in blank syringes from that of test substrates.
In vitro dry matter degradability (IVDMD)
After 24 h of incubation period, the content of the syringes was transferred to 500 mL spoutless beakers, which was extracted in 100 mL of neutral detergent solution (NDS) by boiling for one hour, followed by filtration on pre-weighed gooch crucibles (G1), and washing in hot distilled water and acetone to recover true undigested residue as per the method of Van Soest et al. (1991). Crucibles with undigested residue were dried at 100℃overnight and weighed to determine true undigested residue. Residue was ashed at 500℃ for 3 h to determine true undigested OM, which was corrected for the appropriate blanks. At 24-h post inoculation, a set of samples was determined In vitro true digestibility of truly degradable dry matter (TDDM) and truly degradable organic matter (TDOM) according to Van Soest and Robertson (1985) as the difference between OM incubated and the undigested OM recovered in the residue of ND extraction. The microbial biomass production (MBP) and partitioning factor was calculated as per the method of Blummel et al. (1997).
MBP = Substrate truly degraded - (gas volume*stoichiometrical factor) (1)
For roughages, the stoichiometrical factor was 2.20
Estimation of volatile fatty acid
After 24 h incubation 1 mL of the supernatant of each syringe content was taken in a micro centrifuge tube containing 0.20 mL metaphosphoric acid (25%, v/v) to analysis the total volatile fatty acids (TVFA), acetate, propionate and butyrate. The mixture was allowed to stand for 2 h at room temperature and centrifuged at 5,000 × g for 10 min to get clear supernatant. The supernatant (1 μL) was injected into gas chromatograph equipped with flame ionization detector and glass column packed with chromosorb as per the method described by Cottyn and Boucque (1968).
Statistical analysis
All data were statistically analysed by using Statistical Package for Social Sciences software package (SPSS version 20.0, IBM, Chicago, USA) following one-way analysis. All the observations were recorded at 95% (p < 0.05) level of significance.
Results and Discussion
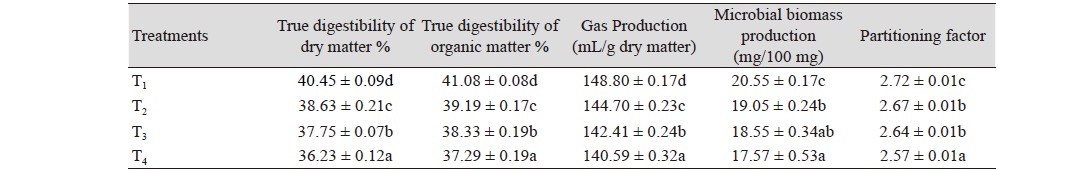
The data pertaining to TDDM, TDOM, gas production, MBP and partitioning factor was statistically analysed, and the mean values are presented in Table 2. The data pertaining to VFA production are presented in Table 3. The TDDM value among dietary treatments varied between 36.23 (T4) and 40.45% (T1); and that of TDOM varied between 37.29 (T4) and 41.08% (T1). The TDDM and TDOM values in control group was higher (p < 0.05) than those of T2, T3, and T4. The TDDM and TDOM values in T2 group was higher (p < 0.05) than those of T3 and T4. The TDDM and TDOM values in T3 group was higher (p < 0.05) than that of T4. The results revealed that inclusion of AFB1 100 ppb in feed significantly decreased the DM and OM degradability compared to that of control. The results further revealed that inclusion of AFB1 to the feed showed that significant decrease in the DM and OM degradability. This result was in agreement with that of Mojtahedi et al. (2013) who also reported that IVDMD decreased significantly (p < 0.05) with inclusion of AFB1 in culture medium, so that the lowest and the highest IVDMD values were observed in treatments with 900 and 0 ng/ml AFB1, respectively (0.54 vs. 0.68). They further reported that decreasing IVDMD with AFB1 addition can be attributed to compromised ruminal function by reducing fibre digestion and volatile fatty acid production (Fehr and Delage, 1970; Helferich et al., 1986a; 1986b). Similarly, Westlake et al. (1989) reported that IVDMD of alfalfa hay was reduced by 50% with inclusion of 1 μg/mL AFB1. However, some studies reported no effect of AFB1 on In vitro dry matter disappearance of hay (Pettersson and Kiessling, 1976; Jiang et al., 2012). Yeanpet et al. (2018) also reported that IVDMD and organic matter digestibility were not significantly affected by AFB1.
The gas production value in control group (T1) was higher (p < 0.05) than those of T2, T3, and T4. The gas production value in T2 group was higher (p < 0.05) than those of T3 and T4. The gas production value in T3 group was higher (p < 0.05) than that of T4. The results indicated that addition of AFB1 at 100 ppb level in wheat straw significantly decreased the gas production compared to that of control. The study also indicated that incorporation of aflatoxin to the feed at any level (100 to 300 ppb) resulted in significantly decreased gas production. The potential extent of gas production was significantly different among treatments (p < 0.05), of which the AFB1-containing diets was lower than the control group. This result was similar to previous studies (Jiang et al., 2012; Mojtahedi et al., 2013) where the asymptotic gas production numerically deceased by increasing AFB1 dosage. Mojtahedi et al. (2013) reported that by increasing the level of AFB1 from 0 to 900 ng/mL, the gas production rate decreased from 0.071 to 0.051 and cumulative gas production decreased from 196.4 to 166.0 mL/g DM, respectively. Similarly, Jiang et al. (2012) and Helferich et al. (1986a; 1986b) also reported that the gas production parameters were reduced when AFB1 was added. These depressions in the gas production suggest that microbial populations are altered by AFB1 contamination. With regard to MBP, the value in control group (T1) was higher (p < 0.05) than those of T2, T3, and T4. The MBP value in T2 group was higher (p < 0.05) than that of T4. The MBP value between T2 and T3; and between T3 and T4 did not vary significantly. The results of present investigation revealed that inclusion of AFB1 to the feed in significantly decreased the MBP than control.
The partitioning factor is defined as the ratio of substrate truly degraded In vitro (mg) to the volume of gas (mL) produced by it. A feed with higher partitioning factor means that proportionally more of the degraded matter is incorporated into microbial mass, i.e., the efficiency of microbial protein synthesis is higher. Roughages with higher partitioning factor have been shown to have higher dry matter intake (Harikrishna et al., 2012). The partitioning factor among various dietary treatments varied between 2.57 (T4) and 2.72 (T1). The partitioning factor value in control group (T1) was higher (p < 0.05) than others. The partitioning factor value in T2 and T3 groups was higher (p < 0.05) than that of T4. The partitioning factor value between T2 and T3 groups did not vary significantly. The results of present investigation revealed that inclusion of AFB1 to the feed significantly decreased in the partitioning factor value compared to control.
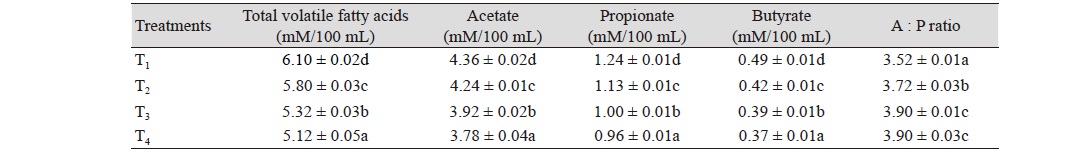
The TVFA, acetate, propionate and butyrate value in control group (T1) was higher (p < 0.05) than those of T2, T3, and T4. The TVFA, acetate (A), propionate (P) and B value in T2 group was higher (p < 0.05) than those of T3 and T4. The TVFA, A, P, and B value in T3 group was higher (p < 0.05) than that of T4. The acetate : propionate (A : P) ratio in control group (T1) was lower (p < 0.05) than those of T2, T3, and T4. The A : P ratio in T2 was lower (p < 0.05) than those of T3 and T4. The A : P value between T3 and T4 groups did not vary significantly. The results revealed that inclusion of 100 ppb AFB1 in feed significantly decreased the TVFA, A, P, and B production compared to control. The results further revealed that inclusion of aflatoxin to the feed at any level (100 to 300 ppb) resulted in significant decrease in TVFA, A, P, and B production. This finding of reduced VFA concentration was in agreement with Jiang et al. (2012) who also reported that the VFA concentration decreased with the increase of AFB1 dose level. Cellulose degradation, VFA production, ammonia production, and proteolysis were decreased by AFB1 at 0.2 - 0.8 mg/kg body weight in acute bovine aflatoxicosis (Cook et al., 1986). Also, the production of VFA irrespective of substrate was inhibited by the increasing dose levels of AFB1, which was consistent with the reduction in the asymptotic gas volume. The suppression of VFA, gas production and ammonia N implicated that microbial activity was inhibited regardless of substrate used. Contrary to this, Edrington et al. (1994) found no differences in ruminal VFA concentrations in growing lambs fed 2.5 mg AFB1 per kg diet. Helferich et al. (1986a) also reported that AFB1 at 60 - 600 ppb did not influence the production of VFA in steers. In another experiment, ingestion of 0.714 µmol AFB1 per animal did not influence the ruminal VFA production in lactating goats (Helferich et al., 1986b). Although data from the literature are inconclusive.
Conclusion
It was concluded that AFB1 contamination of feed (wheat straw) at 100 to 300 ppb levels significantly affected the In vitro rumen fermentation in terms of reduced truly degradable dry matter, truly degradable organic matter, gas production, microbial biomass production, partitioning factor, TVFA concentration and increased A : P ratio.