Introduction
Minerals make up a small but vital percentage of swine diets with profound impacts on animal health, growth performance, feed cost and the general environment. Calcium and phosphorus are the two most abundant minerals in the body, and they exist largely (about 98%) in the form of carbonated hydroxyapatite (Ca10(PO4)6(OH)2) in the skeleton (Mahamid et al., 2010). They are required for a wide variety of functions ranging from the maintenance of the skeletal structure through bone mineralization, lean tissue deposition, blood buffering, energy utilization to many other metabolic roles (Pravina et al., 2013; Oster et al., 2016; Murshed, 2018). It is well established that perhaps their most important role in the body is the development and maintenance of the skeletal system and that due to antagonistic mineral interactions, their accumulation in the skeletal tissue is interdependent of each other (Létourneau-Montminy et al., 2015). Therefore, an oversupply or deficiency of one mineral will affect the utilization of the other (Gonzalez -Vega et al., 2016).
Ca and P nutrition for swine production has been determined to be heavily reliant on; (i) sufficient supply of either element in a utilizable form in the diet, (ii) maintenance of the required ratio of Ca and P and (iii) the provision of enough vitamin D (Peo, 1991; NRC, 1998, 2012). Corn-soybean meal diets are the mainstay for modern-day feed formulations. However, they are characteristically low in calcium as compared to animal sources (NRC, 2005), in addition to the fact that 20 - 30% of Ca in plant tissues is bound to oxalate, thus it is relatively unavailable (NRC, 2001). The majority of phosphorous on the other hand is also bound to phytate (Ravindran et al., 1994; Pallauf and Rimbach, 1996). This renders the phytate-bound P (PP) unavailable for utilization without the intervention of microbial phytase (Selle and Ravindran, 2007).
The use of inorganic mineral sources or animal sources presents a viable option to provide sufficient required amounts of Ca and P in diets but such an approach could negatively impact the environment due to P excretion in manure; costly feeds and lastly, it could lead to the depletion of global reserves of rock phosphate (Kiarie et al., 2013). Alternatively, the use of exogenous enzyme systems such as phytase presents a better approach to alleviating P deficiency due to phytate with reduced environmental effects. It has been shown to improve the digestibility of P up to 60 - 80% in a dose-dependent manner (Selle and Ravindran, 2008). The improvement in P digestibility consequently results in a direct decrease in P excretion to the environment (Singh, 2008; Kiarie et al., 2016). Phytase use has also been attributed to other “Extra-phosphoric effects” such as the improved digestibility of phytate-bound nutrients including Ca, Na, amino acids, and energy (Ravindran et al., 2008; Walk et al., 2016).
Vitamin D on the other hand plays a vital role in skeletal growth and development since it is actively involved in the regulative mechanism for calcium and phosphorus homeostasis (Dittmer and Thompson, 2011; Tousignant et al., 2013). It additionally takes part in the development and functioning of the immune system (Adam and Hewison, 2010; Lopez et al., 2020). Vitamin D can be obtained from the diet either as vitamin D2 or vitamin D3. Pigs have been shown to readily utilize the latter in the form of 25-hydroxy-cholecalciferol (25-OH-D3) also known as calcidiol which is the main circulating form of vitamin D in the body (Horst et al., 1981). The 25-OH-D3 that is formed in the liver proceeds to the kidney where it is hydroxylated to a Ca and P mobilizing hormone namely 1, 25 dihydroxy-cholecalciferol (1, 25-(OH)2-D3). This process is strongly regulated by the need for either Ca or P (DeLuca, 1976a, 1976b). Moreover, according to DeLuca (1979), the synthesis of vitamin D in the body can also be through exposure to sunlight. However, with modern shifts in swine production towards intensification and the use of confined settlements, vitamin D-related rickets has been reported in farms with negative implications such as weakness, lameness, bone fractures or sudden death (Madson et al., 2012). Therefore, adequate vitamin D supplementation in diets could become vital especially for confined piglets since they have a rapid growth rate and are innately born with low levels of plasma vitamin D3 (Horst and Littledike, 1982).
Thus, the current study investigated the influence on weaned piglet growth performance brought about by feeding diets that had different levels of Ca and P with or without supplemental use of super dose phytase or vitamin D3 over three weeks post-weaning. Experiment 1 determined the impact of using diets that had reduced levels of Ca but were sufficient in P with or without supplemental phytase use. Experiment 2 on the other hand, assessed the impact on piglet growth performance brought about by using three different Ca/P ratios with or without the addition of vitamin D3.
Materials and Methods
The Animal Care and Use Committee of Dankook University reviewed and approved the experimental protocol utilized in both studies (Protocol No. DK-1-2030-1 and DK-1-2030-2). The pigs used in both experiments were the offspring of three-way crossbred LYD (Landrace × Yorkshire dam × Duroc Jersey sire) boars. Both experiments were conducted in mechanically ventilated and environmentally controlled facilities at the experimental farm of Dankook University, Sejong farm, South Chungcheong province.
Animals, housing, diets and feeding
Experiment 1
A total of one hundred and twelve weaned piglets were used to determine the impact of using diets that had reduced levels of Ca but sufficient in P on growth performance with or without supplemental phytase. Piglets were weaned at day 21 and fed a standard piglet creep feed that met all standard nutrient requirements for one week. From d 7 post-weaning (d 1), piglets were weighed and in such a way that the initial average body weight was 8.26 ± 0.25 kg, they were then allotted to one of the four treatments in a completely randomized design. Four piglets per pen and seven replicate pens per diet were used.
The Experimental Animal Allotment Program (Lindemann and Kim, 2007) was used to allot the pigs to the experimental diets. They were raised in pens (1.6 × 1.8 m) that had fully slatted floors and were fitted with a feeder and a nipple drinker for ad-libitum access to feed and water. The temperature was maintained at 28℃ for the first week and was lowered by 1℃ for every following week thereafter. All ingredients used in the study were chemically analyzed for Ca and P then used at a commercial feed mill (DH Vital Feed, Pyeongtaek, Korea) to manufacture the diets used in this study.
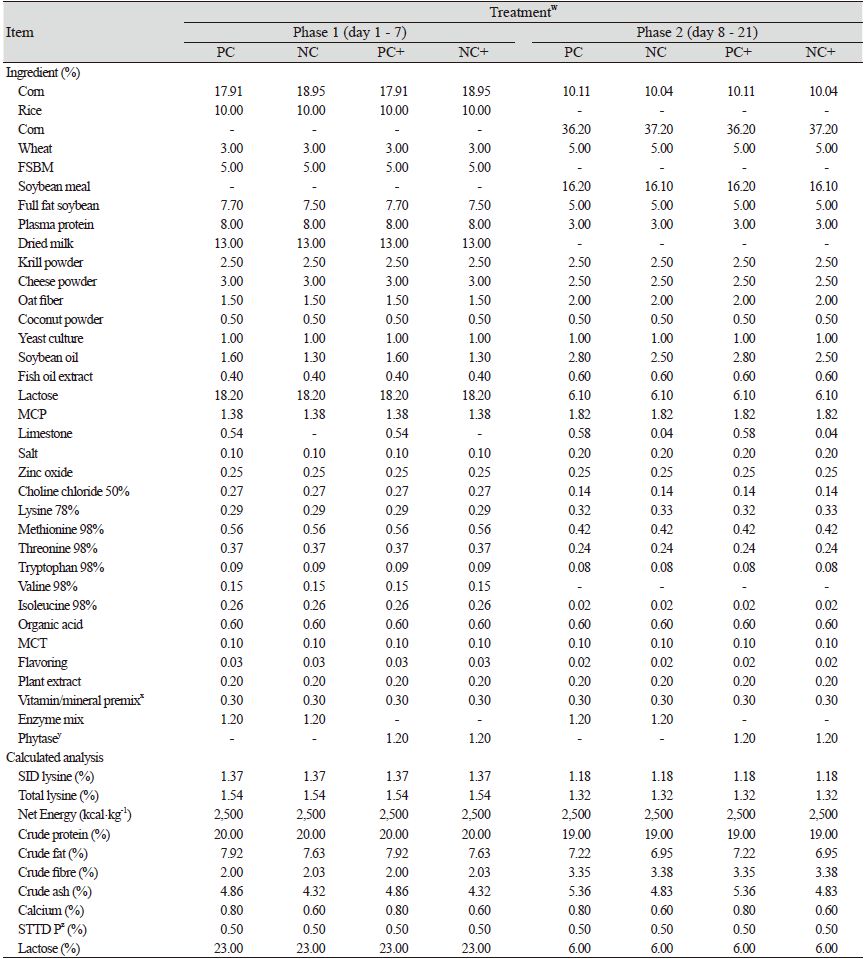
Dietary treatments were corn-soybean meal-based and were formulated to meet or exceed the recommended nutrient levels as per NRC (2012), except for Ca (see Table 1). Monocalcium phosphate monohydrate (MCP) and limestone were added to obtain the calculated total Ca : standardized total tract digestible (STTD) P ratios. The use of MCP to exceed the P requirement ensured that the study evaluated the response to phytase irrespective of meeting the P requirement for the pigs.
The diets included a positive control (PC) diet having total Ca : STTD P ratios of 0.80 : 0.50%. The PC diet was formulated to meet or exceed the NRC (2012) total Ca : STTD P standards for piglets weighing between 7 - 25 kg. The NC diet had Ca : STTD P ratios of 0.60 : 0.45%. The last two treatments were the PC and NC together with the commercially available phytase. They are denoted as PC+ and NC+ respectively, in Table 1. All the diets were fed in two Phases of day 1 to day 7 then from day 8 to day 21.
The phytase was super dosed from the industry recommended level of 1,000 Phytase units (FTU)·kg-1 to 2,000 FTU·kg-1 (super dose level). Phytase activity is normally expressed in phytase units (FTU). One FTU being defined as the amount of phytase that liberates one micromole per minute (1 μmol·min-1) of inorganic phosphate from 0.0051 mol·L-1 sodium phytate at temperatures of 37℃ and pH of 5.50 (Engelen et al., 1994). The phytase used was Natuphos® E 5000 (BASF Corporation, Florham Park, New Jersey, USA).
Experiment 2
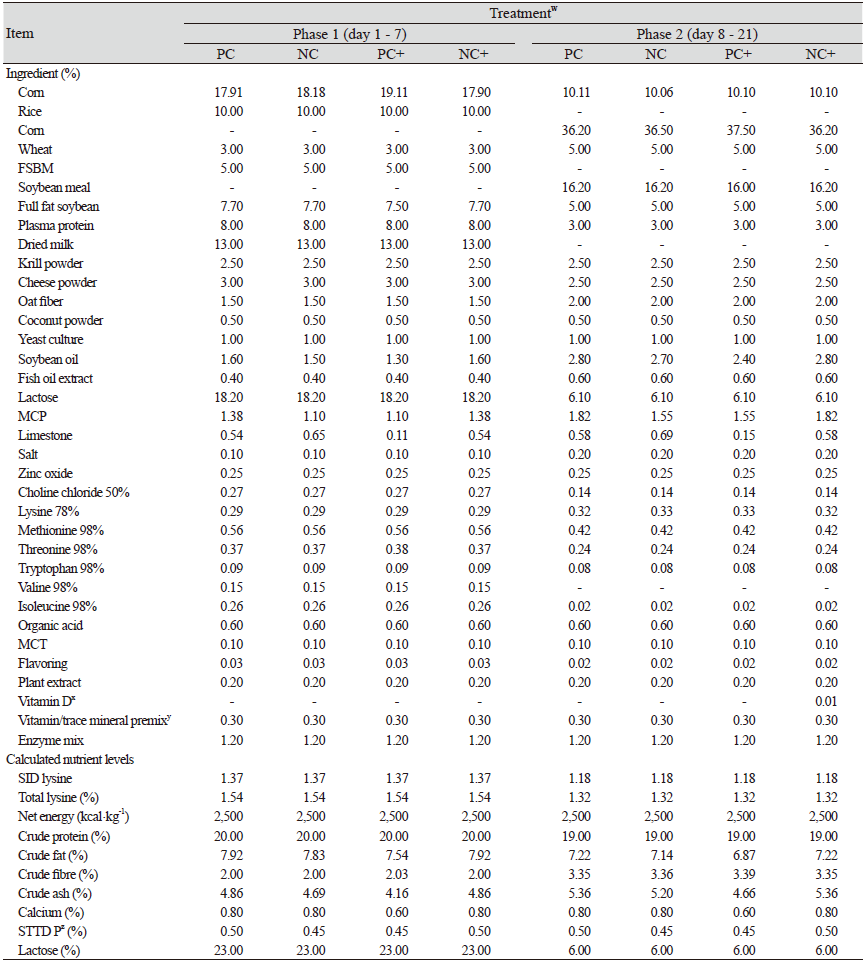
A total of 96 weaned piglets were used to determine the effect on growth performance of using diets that had different Ca : STTD P ratios with or without supplemental use of vitamin D3. With an initial average body weight of 7.44 ± 0.27 kg, the piglets were randomly allotted to one of the four treatments having six replications per diet and four pigs per pen. Similar housing conditions as those in Experiment 1 were used, together with ad-lib access to feed and water.
Treatments included three diets of PC, NC1 and NC2 having total Ca : STTD P ratios of 0.80 : 0.50, 0.80 : 0.45 and 0.60 : 0.45% respectively as shown in Table 2. The PC met all the Ca/P standards for pigs weighing 7 - 25 kg as per NRC (2012); the NC1 diet had reduced levels of P while both Ca and P were reduced for the NC2 diet. The fourth diet (PCV) was the PC supplemented with a commercially available 25-hydroxy-cholecalciferol (25-OH-D3) additive offered at 0.01% (2,000 IU·kg-1). The additive used was Rovimix Hy-D® (DSM Nutritional Products Ltd., Kaiseraugst, Switzerland).
Growth performance and statistical analyses
The feed samples were analyzed for dry matter, crude protein, ether extract, crude fibre, crude ash, calcium, phosphorous and amino acids as per the procedures detailed in AOAC (2005). The feed allotments and health status of the pigs were monitored twice daily throughout the entire experimental period for disease problems, mortality, and feed insufficiency. The body weight (BW), and feed consumed were measured weekly (d7, 14, and 21). Using the body weight and feed consumed data, the average daily gain (ADG), average daily feed intake (ADFI) and finally, the feed conversion ratio (FCR) to depict the efficiency of converting feed to lean muscle mass was calculated (Oketch et al., 2021). The growth performance data was then analyzed using the General Linear Model (GLM) procedures in the SPSS software package (Version 26, IBM SPSS, Chicago, USA). One-way ANOVA analysis was performed to ascertain the difference between the test groups and P-value < 0.1 was used to indicate significant results. The tukey’s multiple range test was used to compare the significant differences between the varying pairs of means. A further analysis using two-way ANOVA was conducted for Experiment 1 to determine the interactive effect between the different Ca : P ratios and super dose phytase.
Results
Experiment 1
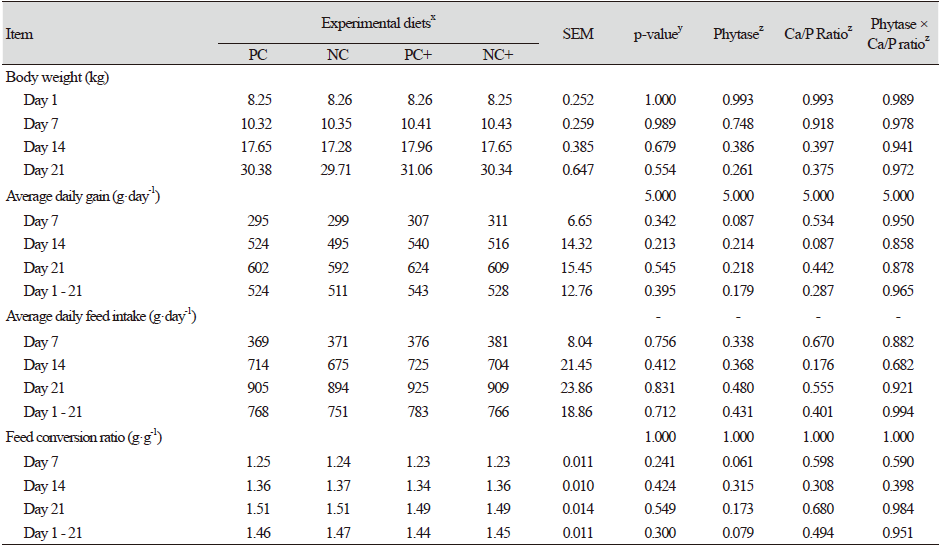
The effect of lowering the Ca in P adequate diets in addition to, the impact of supplementing phytase at a super dose level in such diets was assessed. All pigs subjected to the experiment willingly consumed their diets and no disease incidences were recorded. Results are presented in Table 3. No significant differences in the body weight, ADG, ADFI and FCR were exerted by lowering the Ca while retaining STTD P ratios as well as super-dosing phytase as per the one-way aNOVA analysis. At the end of phase 1, pigs fed the NC (Ca : P of 0.60 : 0.50%) diet without phytase relative to the PC diet, consumed more feed and tended to gain more weight with a 9.02% markedly lower FCR though no significant differences were observed. After phase 1, there was a shift with an improved (p > 0.1) ADG and ADFI being recorded with the use of the PC diet relative to the NC diet. A substantially lower FCR (p = 0.241) was also recorded.
The addition of phytase at a super dose level in the PC+ and NC+ diets resulted in higher ADG, ADFI and lower FCR values as compared to the PC and NC diets although the results were not statistically significant. The NC+ diet led to the improved values recorded for the ADG (p = 0.342) which was the result of the higher values of ADFI (p = 0.576) that were noted at the end of week 1. However, by the end of the three weeks (day 21), better numerically higher values were observed with the PC+ (PC + phytase) diet for the ADG and the ADFI although the results were similarly not of statistical relevance (p < 0.1)
A further analysis using two-way ANOVA was conducted to determine the interactive effect of the different Ca : P ratios alongside the super dosing effect on the indices measured. Results showed that significant improvements (p < 0.1) were recorded for the overall FCR (day 1 - 21) with the use of phytase at a super dose level even though no major interactive effects (P < 0.1) were exerted by the phytase and Ca/P ratios on the ADG, ADFI, and the overall body weights.
Experiment 2
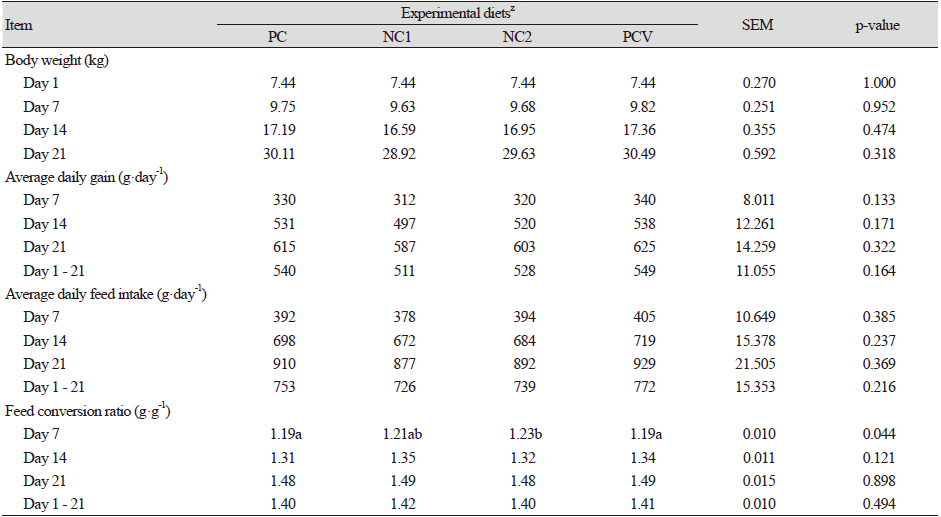
The impact of varying the Ca : STTD P ratios and the effect of providing supplemental vitamin D3 was investigated. The initial BW (7.44 ± 0.27 kg) was the same across all the treatments. After each successive phase, the BW and feed consumed were measured and the ADG, ADFI and FCR were subsequently calculated. The results for Experiment 2 are presented in Table 4. Better growth performance was recorded in pigs fed the PC diet (Ca : STTD P of 0.80 : 0.50%) relative to the NC1 (Ca : STTD P of 0.80 : 0.45%) and the NC2 (Ca : STTD P at 0.60 : 0.45%) diet, but the effects were not of statistical significance.
Throughout the entire 6-week period, birds fed the PC diet consumed more feed, gained more weight and were more feed- efficient as depicted by the lower FCR when compared to the negative control diet (NC1 and NC2). Within the negative control diets (NC1 and NC2), markedly higher values for ADG and ADFI (p > 0.1) were noted with the NC2 diet relative to the NC1 diet. Feeding the PCV diet (PC + 25-OH-D3) similarly, led to numerical increases in the ADG, ADFI and overall body weight as compared to all the other treatments even though the results were not statistically significant.
Discussion
Experiment 1
The impact on weaned piglet growth performance brought about by reducing Ca from the standard requirements while supplying P in marginally excess amounts was assessed. Results of Experiment 1 confirmed that the use of the PC diet (Ca : STTD P 0.80 : 0.50%) led to numerically higher ADG, ADFI, feed conversion ratios and overall better growth performance. It was formulated to meet or exceed the NRC (2012) total Ca : STTD P standards for piglets weighing between 7 - 25 kg. Subsequent lowering of Ca to 0.60% while maintaining the STTD P at 0.50% for the NC negatively affected the growth performance beyond day 7.
According to Becker et al. (2020) and Delezie et al. (2015), for maximized growth performance and skeletal development, emphasis should be placed on maintaining the adequate required levels of Ca and P in diets since Ca reduction to lower levels could hamper both processes. Due to antagonistic mineral interactions, the accumulation of Ca and P in the skeletal tissue is interdependent of each other and an oversupply or deficiency of one mineral will affect the utilization of the other (Létourneau-Montminy et al., 2015; Gonzalez-Vega et al., 2016). Lagos et al. (2019b) suggested that if P is included in excess, then Ca has to also be in excess and vice versa is also true. The same notion applies even with phytase supplementation. Since the beneficial impact induced on digestibility of phytate bound P (PP) may be challenged by extra urinary losses of P that have been recorded when dietary Ca is low (Létourneau-Montminy et al., 2012; Gutierrez et al., 2015).
It has been recorded that the major role of Ca and P in the body is in the development and maintenance of the skeletal system. It has also been observed that although increases in the content of urine P have been associated with the supply of higher than recommended levels of P, the femur Ca and P content also seemed to increase with elevated levels of mineral provision (Gutierrez et al., 2015). This could suggest that the requirement to maximize the main role of Ca and P (bone mineralization) could be over and above the requirement needed to maximize growth performance (Stein et al., 2008; Saraiva et al., 2012; Lagos et al., 2019b). Therefore, to mitigate potential underfeeding of P and the subsequent suboptimal bone development especially when using higher doses of phytase in diets, the use of additional dietary P in such diets has been suggested (Wu et al. 2019).
However, phosphorus is the third most expensive nutrient after energy and protein (Tahir et al., 2012; Patience, 2017). The majority of plant origin feed ingredient P (> 65%) is also in the form of phytate which chelates P (Ravindran et al., 1994; Pallauf and Rimbach, 1996) thus rendering the phytate-bound P (PP) unavailable for utilization without the intervention of microbial phytase (Selle and Ravindran, 2007). The use of diets that are low in P have also been associated with lameness in animals, reduced growth performance with lower ADG and ADFI in addition to a possible sub-optimal bone development (Ekpe et al., 2002; Létourneau-Montminy et al., 2012).
Therefore, P supplementation through inorganic sources such as defluorinated phosphate, monosodium phosphate, monocalcium phosphate mono- and dicalcium phosphate could be plausible (Létourneau-Montminy et al., 2012). Nevertheless, unlike calcium, excess P use has been subject to environmental concerns due to mineral excretion in addition to its overall impact on feed cost. Therefore, it is common practice to incorporate the use of the enzyme phytase to hydrolyze the anti-nutritional factor phytate and release phytate phosphorous (PP) rather than incorporate the use of inorganic phosphates (Bedford and Schulze, 1998; Choct, 2006; Woyengo and Nyachoti, 2011; Gadde et al., 2017). Kiarie et al. (2013) reported that phytase dominates the exogenous feed enzymes market with a lion 60% share.
Diets that are sufficient in Ca but lower in P have traditionally been used for assessing phytase even though as reported by Zanu et al. (2020), the use of the marginally higher Ca in such diets precipitates phytate leading to the formation of insoluble Ca-phytate complexes. This could impede the effectiveness of evaluating phytase by reducing the enzymes’ mucosal activity and ileal phytate degradation (Applegate et al., 2003). As a result, it could hypothetically be more physiologically relevant for the assessment of phytase to be conducted using diets that meet or exceed the recommended levels of P (Olsen et al., 2019). However, there is a paucity of information on phytase use in P adequate diets.
Therefore, additional analysis to investigate the effect of supplementing phytase at a super dose level in the P adequate diets was determined. This was based on the hypothesis that the functional relevance of supplementing phytase at super dose levels could be better evaluated in such diets. The administration of super dose phytase above the level required to meet the phosphorus requirement resulted in significant improvements for the ADG and the FCR. Similar improvements with the addition of phytase in P adequate diets were first observed by Beers and Jongbloed (1992) although conflicting results have been recorded by Gourley et al. (2018) for the ADG and ADFI value. The improved growth performance brought about by super dosing phytase could have seemingly resulted from the greater phytate hydrolysis (Ravindran, 2013).
“Super-dosing” incorporates the use of higher than recommended levels of phytase to achieve greater results (Humer et al., 2015). The greater hydrolysis of phytate due to super dosing could generate lower esters of IP6 including IP5, IP4, IP3, and IP2. IP2 is digestible by the gastric alkaline phosphatase in a process that results in the release of inositol, a nutrient that has been suggested to be growth-promoting in nature (Cowieson et al., 2011; Bedford and Rousseau, 2017). However, varied results have been recorded with phytase supplementation at super dose levels with Holloway et al. (2018), Kies et al. (2006), Zeng et al. (2014), and Adhikari et al. (2015), reporting positive improvements while Flohr et al. (2014a) observed no benefits of super- dosing phytase.
Super dosing could also bring into light the extra-phosphoric effects of phytase. The present study reported significant improvements were noted for the FCR with super dosing of phytase, thus supporting the findings of Walk et al. (2013) who proposed that when P is not limiting, the phytase response could be limited to improvements in the feed to lean muscle conversion ratios. Super dosing could also facilitate the release of the other limiting components of the diets excluding P (Kies et al., 2001; Walk et al., 2013). In this instance, Ca could be the main candidate since it is a cation (+ve) and can be effectively bound to phytate which is a negatively charged ion (Ravindran et al., 1995). Since 1 phytate molecule binds to 5 Ca molecules (Selle et al., 2009), Ca digestibility has been shown to improve with increasing levels of phytase addition (Kies et al., 2006). Therefore, as stated by Cowieson et al. (2011) super-dosing phytase could be restorative, leading to a proportionate release of the limiting mineral.
However, it could be worth noting that better growth response was observed with the PC+ (PC plus phytase) diet as compared to the NC+ (NC plus phytase) treatment. This could have resulted from a possible Ca relative insufficiency in the NC+ diet. Similar results have been reported by Holloway et al. (2018) whose results showed that super dosing phytase in a marginally deficient diet was no greater than a diet that is almost or fully balanced for all nutrients. This suggests as noted by Driver et al. (2005) that if super dose phytase is used in P-sufficient diets, an improved response could be obtained at higher levels of Ca. The reverse could be true that if low P is used in phytase supplemented diets then the Ca also be reduced. This is because it is important to maintain the required balance between the two minerals and avoid the formation of insoluble Ca-phytase complexes that could be formed in phytase experiments having low P and high Ca (Qian et al., 1997; Bougouin et al., 2014; Dersjant-Li et al., 2015). Care should be taken to avoid insufficiency while lowering Ca and P lest growth performance and bone mineralization could be compromised.
Experiment 2
The impact brought about by different Ca : STTD P ratios were to be determined alongside the effect that a commercially available vitamin D3 source could have on weaned piglet growth performance. Our results suggested that the feeding of the PC diet with the Ca : STTD P ratio set at 0.80 : 0.50% tended to result in a numerically higher ADG, ADFI with a desirably reduced FCR. This performance was relative to the NC1 diet where the STTD P was marginally reduced to 0.45%, and the NC2 diet where both the Ca and STTD P ratios were slightly reduced to 0.60 and 0.45% respectively (see Table 5). The relatively improved better performance with the PC diet could be explained by a possible provision of Ca and P over and above the requirements as per the NRC (2012) requirements. It is worth noting that slightly better performance that was not of statistical significance was recorded with the NC2 diet that had even lower Ca and P levels compared to the NC1 diet. It is not yet clear what led to this response.
Plant-based diets in form of corn-soybean meals are the mainstay for modern-day feed formulations. Unfortunately, they are characteristically low in calcium as compared to animal sources (NRC, 2005). Additionally, 20 - 30% of Ca in plant tissues is bound to oxalate, thus it is relatively unavailable (NRC, 2001). Therefore, it is common practice to incorporate the use of inorganic feedstuffs such as mono- and dicalcium phosphate, ground limestone and calcium chloride. Their excessive use, because they are relatively inexpensive, has not subject to environmental concerns (Zhang and Adeola, 2017), even though there could be a marginal calcium oversupply in swine diets. Recent research has demonstrated that with excess dietary Ca, unabsorbed Ca interacts with P in the chyme to form insoluble Ca-P complexes, this reduces P absorption with profound impacts on growth performance and bone calcification (Reinhart and Mahan, 1986; Heaney and Nordin, 2002; Stein et al., 2011). Marginally excess Ca even reduces the impact of phytase on the release of P from phytic acid (Selle et al., 2009; Dersjant-Li et al., 2015). The impact is more pronounced when diets are limiting in P (Létourneau-Montminy et al., 2015; Merriman et al., 2017; Wu et al., 2018). Maintenance of the adequate ratios of Ca and P is therefore vital with or without additive use.
Since vitamin D is actively involved in the regulative mechanism for calcium and phosphorus homeostasis, it was hypothesized that the use of a commercially available vitamin D3 source could positively impact weaned piglet growth performance. Our current findings showed that the use of the vitamin D3 source resulted in numerical increases in the ADG, ADFI and overall body weight as compared to the other treatments even though the results were not of statistical significance. Similar results have been reported by Flohr et al. (2014b), whereby vitamin D3 use led to an increase in serum levels of 25-OH-D3 but no major improvements were noted for growth performance and bone mineralization.
Ca absorption from the small intestine is usually carried out by either paracellular and transcellular transport in response to high or low Ca respectively (Bouillon et al., 2003; Bronner, 2003; Pérez et al., 2008). Lagos et al. (2019a) outlined that transcellular transport in response to low Ca is a saturable process that requires energy, Ca-binding proteins (calbindins), and calcium channels such as the transient receptor potential cation channel, subfamily V, member 6 (TRPV6). Calcium is first absorbed through the Ca channels located in the brush border membrane (van de Graaf et al., 2004). It is then bound in the cytosol to the binding proteins that facilitate transport towards the basolateral membrane (Kaune, 1996) where the release of Ca be accomplished via plasma membrane Ca-ATPase activity (Lagos et al., 2019a). The release process is regulated by vitamin D.
Vitamin D goes through two hydroxylation processes for its activation. First is through the action of the hepatic 25-hydroxylase to generate 25-hydroxy-cholecalciferol (25-OH-D3) also known as calcidiol which is its main circulating form (Molin et al., 2017). Thereafter, 25-OH-D3 can be hydroxylated by the renal 1α-hydroxylase into 1, 25- dihydroxy-vitamin D3 (1,25(OH)2D3) also known as calcitriol. It is the physiologically active form of vitamin D, through which the parathyroid hormone or calcitonin maintains Ca and P homeostasis (Dittmer and Thompson, 2011). The activation of calcitriol has been observed to be in response to low plasma Ca levels (Eklou-Kalonji et al., 1999; Fleet and Schoch., 2010), leading to increased absorption of Ca (Lagos et al., 2019a).
Therefore, perhaps the major focus should be on Ca and P balance since vitamin D tends to affect growth performance significantly when the animal is deficient in either Ca or P. It has been suggested that better results of vitamin D3 use in modern swine diets could be achieved in pregnant and lactating sow diets (Witschi et al., 2011). This is because piglets from such sows recorded significantly improved vitamin D status with added benefits on growth performance. Similar results have since been supported by Upadhaya et al. (2021). Additionally, the immaturity of hepatic 25-hydroxylase- the enzyme responsible for converting vitamin D3 into its main circulating form (calcidiol) has been noted in newborns (Hollis et al., 1996).
Conclusion
Generally, it was noted that better growth performance was achieved with Ca : STTD P ratios closer to NRC (2012) standards in the PC diets. Deficiencies and imbalances of Ca and P should be avoided. The overall conclusion of this study highlights the relevance of maintaining the adequate recommended Ca : STTD P ratios while exploiting the use of available feed additives such as phytase at super dose levels for notable improvements in the feed conversion efficiency. No major effects were observed with vitamin D supplementation.
Acknowledgements
The authors acknowledge the support provided by Dodram Pig Farmer's Service CO., Ltd., South Korea.
Authors Information
Oketch Elijah Ogola, https://orcid.org/0000-0003-4364-460X
Jun Seung Choi, https://orcid.org/0000-0003-0199-6682
Jun Seon Hong, https://orcid.org/0000-0003-2142-9888
Yu Bin Kim, https://orcid.org/0000-0001-7720-128X
Shan Randima Nawarathne, https://orcid.org/0000-0001-9055-9155
Myunghwan Yu, https://orcid.org/0000-0003-4479-4677
Jung Min Heo, https://orcid.org/0000-0002-3693-1320