Introduction
The focus of our research has been to screen locally available putative contraceptive plants in order to find ones that could be effectively applied as topical contraceptives. Earlier, herbal contraceptive researches were mostly performed by feeding extracts to experimental animals, followed by investigations of their effect on gonad health, gamete development (Shajeela et al., 2011; Daniyal and Akram, 2015), embryo implantation, litter size (Sharma and Jocob, 2001) or sexual hormonal profile (Atsukwei et al., 2015; Msiska et al., 2021). In contrast, we have been studying the direct effects of the extract on in vitro cultured spermatozoa (Bhandari et al., 2021). Among several functions of spermatozoa that are crucial for fertilization, we have undertaken to study acrosome reaction, viability and membrane damage. These initial assessments can be done in a simple laboratory setting with classical microscopic techniques. The presence or absence of acrosome in spermatozoa is an important indicator that determines whether the spermatozoa are capable of fertilization. Spermatozoa, which precociously lose acrosome (acrosome reacted) long before coming in contact with the zona pellucida or spermatozoa which are non-viable or membrane damaged, cannot fertilize oocytes (Meyers, 2009; see below). Calcium ionophore (Yanagimachi, 1975), heparin (Parrish et al., 1988), various steroid hormones, kinases, and physical treatments (Tarin and Trounson, 1994), platelet-activating factor (Wu et al., 2020), etc. induce a precocious acrosome reaction that would render them immobile (Tateno et al., 2013) and incapable of fertilizing oocytes.
In a previous study, we have observed that the leaf extract of a putative contraceptive herb Artemisia vulgaris significantly reduced fertility in mice, most probably by inducing a precocious acrosome reaction in spermatozoa (Bhandari et al., 2021). The direct conceptive effect of the extract was evaluated by injecting the extract into the vagina and allowing the females to mate. Artemisia extract caused a partial but significant reduction in litter size (Bhandari et al., 2021). Screening herbal extracts in this manner would be the most appropriate method for discovering ideal herbal contraceptives that could be applied topically just before copulation. In the present work, we have studied the contraceptive effects of tuber extract of Dioscorea bulbifera by following a procedure similar to the Artemisia study (Bhandari et al., 2021).
Dioscorea bulbifera (yam), a potential contraceptive herb, is a monocotyledonous, perennial, twinning vine with oval leaves. Its bulbils are axillary, rounded, 5 - 6 cm wide, and its tubers are renewed annually. They are edible in the cultivated varieties but may be toxic when grown in the wild. The plants grow at an altitude of 150 to 2,000 m especially in the lower hills and middle mountains of Nepal. In Nepal, they grow in the wild, and are also cultivated.
Along with scores of bioactive phytochemicals, the tuber and bulbil of Dioscorea contain diosgenin, dioscorin, dioscorine, etc. (Obidiegwu et al., 2020). Diosgenin is a steroidal saponin utilized for synthesizing estrogen and progesterone on an industrial scale (Balandrin et al., 1985). Dioscorin comprises two proteins, a 32 kDa yam storage protein with carbonic anhydrase activity (Hou et al., 1999) and a 200 kDa protein showing an angiotensin I-converting enzyme inhibitory property (Nagai and Nagashima, 2006). Dioscorine is a toxic alkaloid (Broadbent and Schnieden, 1958). Dioscorea bulbifera and other related species are used to reduce the fertility of women in some ethnic communities (Swarnkar and Katewa, 2008; Kamble et al., 2010; Dutta, 2015) or to alleviate their reproductive ailments (Zeng et al., 2018; Obidiegwu et al., 2020). However, its contraceptive effect is yet to be evaluated.
Materials and Methods
Collection of plant materials and extract preparation
Dioscorea bulbifera bulbils were collected from the Dhading area and the tubers were obtained in dried form, collected from the Kathmandu area of Nepal. The plant parts were washed thoroughly and chopped into small pieces, dried at room temperature overnight, and then in an oven at 60℃ for three days. The completely dried plant parts were ground into powder using a grinder.
Extracts were prepared by soaking 100 gm of powder with 300 mL of 80% ethanol (1 : 3 ratio) in a tight dark bottle. The bottles were shaken in a shaker in darkness for three days. The solutions were filtered by using Whatman’s No.1 filter paper and stored at room temperature in dark bottles.
Mouse handling and husbandry
Male and female Swiss albino mice aged 10 - 14 weeks and 8 - 12 weeks respectively, were purchased from the Department of Plant Resources, Thapathali, Kathmandu, Nepal. Polypropylene cages were used to house the mice, maintained at 12 h light: 12 h dark period and 22 - 28℃ temperature. Mice were provided with adequate water and solid pellet food (VRK Nutritional Solutions, Pune, India). All studies involving handling the mice were done in strict accordance with the approved protocol issued by the Ethical Committee of the Central Department of Biotechnology/Tribhuvan University, Kathmandu, Nepal (protocol number CDBT/TU 084/2019).
Media preparation
Growth media, supplements, and buffers were purchased from HiMedia Laboratories, Mumbai, India. For sperm isolation and culture DMEM (Dulbecco’s Modified Eagle’s Medium, D5523, 10 gm·L-1) was used. To the medium were added either sodium bicarbonate (NaHCO3, 3.7 mg·mL-1) for incubating in a CO2 incubator or 25 mM of 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid (HEPES) for culturing in a normal incubator. After adding sodium bicarbonate or HEPES, the pH of the solution was adjusted to 7.2 and then 5 mg·mL-1 bovine serum albumin (BSA) was added. The media were kept warm in a 37℃ incubator.
1.5 mL Eppendorf tubes were used to culture the spermatozoa. To each tube, a calculated volume of the extract stock solution and sodium bicarbonate buffered medium were mixed to make 1 mL of treatment medium. The series of 5 tubes contained 25, 100, 500 (or 400), 1,000, and 2,000 µg·mL-1 of extract concentrations. In the blank control, the extract was excluded and in the vehicle controls, 80% ethanol with volumes equivalent to 1000 and 2000 µg·mL-1 treatment medium, were added. The tubes were equilibrated in a CO2 incubator for 2 h.
Spermatozoa isolation and treatment
While the treatment media were being incubated, spermatozoa were isolated. Male mice were sacrificed by cervical dislocation. Both testis and epididymis were exposed, removed intact, and collected in a Petri dish with a warm HEPES buffered medium. The cauda part of each epididymis was excised and transferred to 1 mL of warm HEPES buffered medium taken in a separate Petri dish. With forceps and scalpel, the epididymis were incised and the sperm content was pushed out of the tissue into the medium. The spermatozoa disperse into the medium in a few minutes which can be expedited by 2 - 3 gentle in-and-out pipetting. The spermatozoa suspensions were taken in 1.5 mL Eppendorf tubes and centrifuged at 300×g for 1 min to remove contaminating red blood cells (RBC) and tissue chunks. The supernatants were transferred to new Eppendorf tubes and quickly placed in the incubator. Sperm motility was checked by microscopic examination and the concentration of spermatozoa was estimated using a hemocytometer (Yi et al., 2021).
Sperm treatment was started by adding a calculated volume of isolated spermatozoa to each tube prepared as described above, making the final concentration equal to 5 × 105·mL-1. The sperm cultures were incubated in CO2 for another 2 h.
Coomassie and fluorescein-labeled peanut agglutinin (FPNA) staining
Coomassie brilliant blue (G-250) was purchased from Sigma-Aldrich (Baden-Württemberg, Germany) and fluorescein isothiocyanate (FITC) peanut agglutinin (FPNA) from Molecular Probes-Invitrogen (Middlesex County, MA, USA). After completion of incubation with extract the Eppendorf tubes were centrifuged at 500 × g for 3 mins. The supernatant was slowly aspirated leaving 100 µL of concentrated sperm suspension and pellet at the bottom. The pellet and supernatant were mixed by gentle pipetting. The treated sperm suspensions were spread on the coverslips. They were left for 3 to 5 mins for the spermatozoa to settle on the coverslips. The spermatozoa attached to the coverslips were fixed in 4% formaldehyde in phosphate-buffered saline (PBS) for 30 mins at room temperature. After the fixation, the coverslips were processed for coomassie staining and FPNA labeling.
Some coverslips were stained with coomassie (0.25 g coomassie G-250, 10 mL of glacial acetic acid, and 90 mL of 25% methanol) for 5 mins, washed quickly with 1x PBS, and left to dry. Then the coverslips with cells side up were attached on slides using dibutylphthalate polystyrene xylene (DPX). The slides were observed under a light microscope with a 100x oil immersion lens. Two hundred sperm cells with or without acrosomes were counted.
For FPNA labeling, spermatozoa attached on coverslips and fixed with formaldehyde were permeabilized by adding 100 µL of the tween (1% Tween20 in PBS) to each coverslip and incubating for 30 mins at room temperature. After removing the tween, 50 µL of 500x diluted FPNA was added to each coverslip which was then incubated in a moist chamber for 1 h. After incubation FPNA was removed, 50 µL of ethidium bromide (EtBr, 100 µg·mL-1) was added and left for 5 mins. EtBr was washed with PBS. For mounting, a small drop of 10% glycerol was taken on a clean slide and the coverslips were mounted with cell side down. The edges of the coverslips were sealed using DPX mountant. The slides were observed with a fluorescence microscope under blue light.
Trypan blue staining and hypo-osmotic swelling test
After incubation, sperm samples were transferred to ice to stop the effect of the extract. The Eppendorf tubes were centrifuged at 500 × g for 3 mins. The supernatants were aspirated and discarded leaving about 20 - 30 µL of the sperm pellet at the bottom. The tubes were immediately put back in the ice bucket and the sperm suspensions and pellets were mixed by gentle pipetting. From each tube, 5 µL of sperm suspension was taken on a slide to which 5 µL of 0.4% trypan blue was added and mixed. The mixture was covered by a coverslip. The number of stained and unstained spermatozoa were counted in a population of 200 spermatozoa.
For the hypo-osmotic swelling test, the extract-treated sperm samples were further incubated in the hypo-osmotic solution (0.735 g sodium citrate, 1.351 g fructose in 100 mL of distilled water). The extract-treated sperm samples were centrifuged at 500 × g for 3 mins. The upper 900 µL of supernatant was discarded. The remaining 100 µL of concentrated sperm suspension was added to 1 mL of hypo-osmotic solution and incubated at 37℃ for 30 mins. After the incubation, the tubes were centrifuged at 500 × g for 5 mins. The supernatant was discarded leaving behind the bottom 100 µL of supernatant and pellet which were mixed. Then slides were prepared by directly adding drops of the suspension to slides and covering them with coverslips. Sperm cells with straight morphology or with bent tails were counted in a population of 200 spermatozoa.
In vivo fertilization
Ten weeks old females and 12-weeks old male mice were used for mating. These mice were acclimatized for three days before the experiment. The vaginal opening of the females was examined every evening to detect their estrous cycles (Byers et al., 2012). On day 1 of the estrous cycle female mice were intravaginally injected with 10 µL of 1000 µg·mL-1 concentrate extract using a 10 µL micropipette. Initially, the injected female mice were paired with male mice for one night and separated in the morning. But this method often resulted in failed copulation, and the females did not become pregnant. Therefore, to ensure fertilization, females were injected with extract in the vaginal opening every evening for three days and left for mating overnight after each injection. Successfully conjugated females were identified by observing the copulation plug. The pregnant mice were separated and kept in different cages. The date was noted, and observations were carried out for up to 30 + 1 days. After parturition, the number of litters was counted. Similarly for vehicle control, female mice in estrous phage were injected with 10 µL of 80% ethanol every evening continuously for three days and left for mating. The blank control involved mating untreated females with males. For each experiment, three sets of mice were used.
Statistical analysis
The statistical calculations of means, standard deviations, significance values (p, t-tests: paired two samples for means) and bar graph drawings were done by using Excel Spreadsheet (Microsoft Office 2013, Redmond, Washington, D.C., USA).
Results and Discussion
The Dioscorea bulbifera tuber extract induced precocious acrosome reaction
The acrosome reaction of mouse spermatozoa was studied by coomassie and FPNA staining. FPNA and EtBr labeling provided an unambiguous distinction between the acrosome- intact and acrosome-lost spermatozoa. FPNA labeled the intact acrosome as a bright green cap-like structure on the dorsal surface of sperm head. The sperm heads appeared bright red when labeled by EtBr (Fig. 1A). Acrosome reacted spermatozoa lacked green labeling while the nuclei were visible as falciform red structures. When stained with coomassie, the unaffected spermatozoa displayed intact acrosome as a crescent-shaped thick blue cap on the dorsal side of head whereas the acrosome reacted spermatozoa lacked such staining (Fig. 1B).
The effect of D. bulbifera tuber extract on sperm acrosomal exocytosis was investigated by conventional coomassie staining method. In the blank control, 20.35% of spermatozoa displayed spontaneous acrosome loss (Fig. 1C). Some spermatozoa are likely to degenerate acrosomes due to physical injury caused by the experimental manipulations. Some might undergo spontaneous acrosome reaction in the native condition without any obvious reason (idiopathic male factor). The incubation of mouse spermatozoa in a culture medium with D. bulbifera tuber extract induced acrosome exocytosis. An extract concentration of 25 µg·mL-1 caused 40.63% acrosomal exocytosis which was significantly higher than the control value (p = 0.039). The percentage of acrosome reacted spermatozoa increased proportionally with increase in concentration of the extract in the medium (Fig. 1C). At an extract concentration of 1,000 µg·mL-1, the proportion of acrosome reacted spermatozoa was 69.5%. An extract concentration of 2,000 µg·mL-1 increased the acrosome reaction slightly (71.14%). This value was not significantly different from the value of the acrosome reaction at 1,000 µg·mL-1 (p = 0.693) despite two-fold increase in extract concentration. Hence, we considered 1,000 µg·mL-1 to be the optimum extract concentration for inducing acrosome reaction in mouse spermatozoa for further experiments. Spermatozoa incubated with 80% ethanol at concentrations equivalent to 1,000 and 2,000 µg·mL-1 extract concentrations (vehicle controls) resulted in 17.71 and 18.41% acrosome reactions, respectively. These observations indicate that the solvent of the extracts did not cause detectable acrosomal reaction.
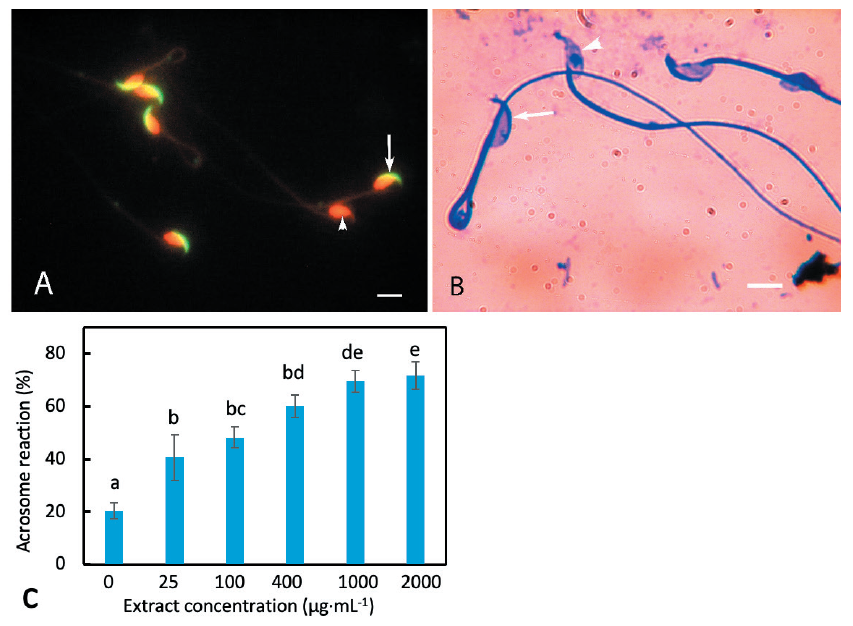
Fig. 1. Effect of the extract on acrosome reaction of mouse spermatozoa. (A) Mouse spermatozoa doubly labeled with fluorescein isothiocyanate (FITC) conjugated lectin from Arachis hypogaea (peanut; PNA) and ethidium bromide (EtBr). Spermatozoa with intact acrosomes displayed crescentshaped fluorescence on the dorsal surface of the heads (arrow). Spermatozoa lacking acrosomes do not display green labeling. Sperm nuclei appeared as red falciform structures due to the EtBr labeling of DNA (arrowhead). (B) Mouse spermatozoa stained with coomassie blue. The acrosome was visible as a thick blue arc on the dorsal surface of the head (arrow). Sperm heads lacking acrosomes were devoid of such staining (arrowheads). (C) Acrosome exocytosis caused by various concentrations of the extract. Increase in concentration of the extract caused a higher percentage of acrosome exocytosis. The columns marked by different letters indicate significantly different values. Scale bars in (A) and (B) indicate 10 μm.
We have also studied the effect of various concentrations of extract on the acrosome reaction using FPNA labeling method. This method also showed results (data not shown) similar to those evaluated by the coomassie staining method (described above; see also, Bhandari et al., 2021).
The acrosome plays crucial roles in fertilization process (Florman and Storey, 1982; Manandhar and Sutovsky, 2007). Spermatozoa that undergo precocious acrosome reaction induced by Dioscorea tuber extract may not be able to fertilize oocytes. The spermatozoa that are capable of fertilization should have an intact acrosome at least until they reach the upper isthmus or ampulla of the female reproductive tract (Hino et al., 2016) or while passing through cumulus cells. Some spermatozoa lacking acrosome may bind to the zona pellucida and penetrate through it (Hino et al., 2016; Jin et al., 2011). However, it is likely that such spermatozoa might possess residual acrosome (Kim and Gerton, 2003; Hirohashi et al., 2011). After the acrosome reaction, spermatozoa cannot live for a long time. In mice, they may live for 4 h (Hino et al., 2016). Spermatozoa that lose acrosome precociously due to the effect of Dioscorea extract in the lower uterus/vagina would not be able meet the oocytes by swimming through the oviduct and isthmus within 4 h. Moreover, the spermatozoa are rendered immotile after treatment with acrosome reaction inducer (Tateno et al., 2013).
The Dioscorea bulbifera tuber extract caused viability loss and membrane damage in mouse spermatozoa
Sperm viability was studied by Trypan blue staining. This dye is permeable into dead spermatozoa, possibly because they usually have damaged membrane, resulting in blue staining of the sperm heads. The stain cannot penetrate live spermatozoa and they, therefore, remained unstained or lightly stained (Fig. 2A). Similar to the acrosome reaction, the addition of extract into the incubation medium caused a viability loss of the spermatozoa. Untreated spermatozoa showed 4.86% death that increased to 9.27% when exposed to the extract at a concentration of 25 µg·mL-1 (Fig. 2B). The effect was thus not significant (p = 0.12). There was a proportional increase of dead spermatozoa with an increase in the concentration of the extract. At 1,000 µg·mL-1 concentration the percentage of non-viable sperm was 33.62%, which was significantly higher than the control value (p = 0.03).
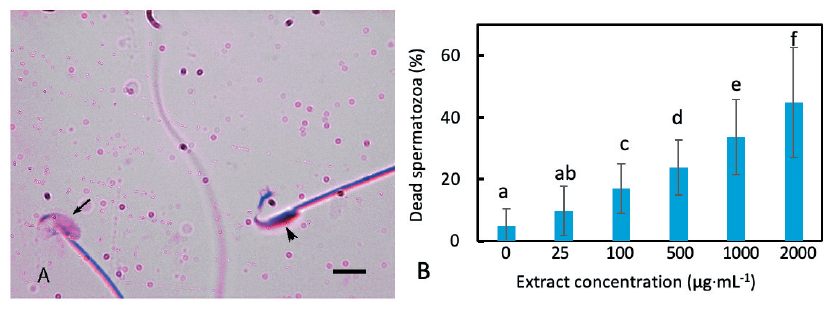
Fig. 2. Effect of the extract on mouse sperm viability. (A) Extract treated mouse spermatozoa stained with Trypan blue. Live spermatozoa were unstained (arrow) while dead spermatozoa displayed deep blue staining in the heads (arrowhead). (B) Loss of sperm viability when incubated in various concentrations of the extract. The percentage of dead spermatozoa increased with the increase in extract concentration. The percentage increase of dead spermatozoa at 1,000 μg·mL-1 was significantly different from that of the control value. The columns marked with different letters are significantly different. A scale bar in (A) indicates 10 μm.
The integrity of the plasma membrane is an important criterion to be considered in order to evaluate the structural and functional state of spermatozoa and their ability to fertilize oocytes. To assay the membrane damage, spermatozoa were incubated in a hypo-osmotic solution for a brief period. Spermatozoa that have an intact membrane imbibe water by endosmosis and swell up (become turgid) resulting in a curvature of the tail or bending at the head-neck or midpiece-tail joints. If the spermatozoa have a damaged membrane the solution can move freely in and out and there will be no turgidity. Therefore, they remain straight.
The incubation of spermatozoa in a medium containing the extract caused membrane damage in spermatozoa as shown by the hypo-osmotic test (Fig. 3A). The higher the concentration of extract, the higher was the rate of membrane damage (Fig. 3B). However, the effect of extract causing membrane damage was remarkably low in comparison to its effect on the acrosome reaction or viability loss. At an extract concentration of 1,000 µg·mL-1, the proportion of membrane damage was 20.5% which was significantly different from the control value (p = 0.002).
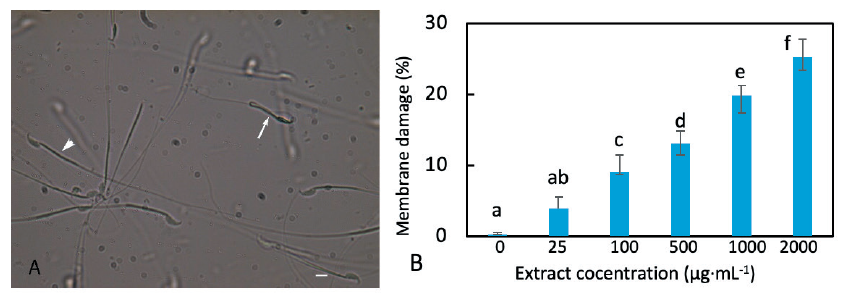
Fig. 3. Effect of the extract on the mouse sperm membrane. (A) Extract treated mouse spermatozoa incubated with the hypo-osmotic solution. Live spermatozoa were affected by the hypo-osmotic solution causing various deformations including the bending and folding of the tail (arrow) while membrane-damaged spermatozoa were not affected, displaying a straight tail (arrowhead). (B) The percentage of spermatozoa with damaged membranes increased proportionally with an increase in the concentration of extract. The columns marked with different letters are significantly different. A scale bar in (A) indicates 10 μm.
The rate of induced viability loss and membrane damage was remarkably less compared to the acrosome reaction. This would mean that some acrosome reacted spermatozoa are live and have an intact membrane boundary. Acrosome reaction caused by the extract seems to be a physiological process rather than a traumatic injury. The sperm heads are still protected by the inner acrosomal membrane, closely apposed to the nuclear membrane even after vesiculation of the plasma and outer acrosomal membranes (Manandhar and Toshimori, 2001). Apparently, the Dioscorea extract affected spermatozoa in a manner similar to the calcium ionophore. Spermatozoa are immobilized by the calcium ionophore, but some of them can recover and fertilize oocytes after the calcium ionophore is removed (Tateno et al., 2013).
The Dioscorea bulbifera tuber extract completely eliminated fecundity in mice
The female mice injected with the extract intravaginally before copulation showed a complete loss of fertility. The blank control females gave birth to more than 9 litters while females injected with ethanol (vehicle control) produced 8 litters (Table 1).
Table 1. Effect of the Dioscorea bulbifera extract on mouse fertility.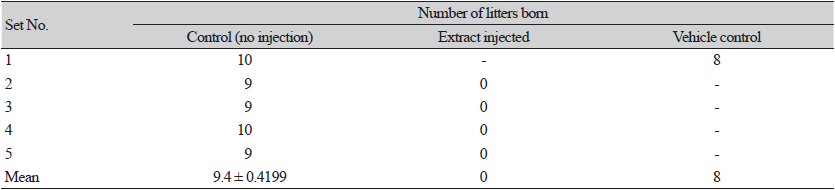
|
|
The female mice were injected with 10 μL of 1,000 μg·mL-1 extract and conjugated with males (see Materials and Methods). |
Since the extract is present in the vagina at the time of sperm deposition, infertility in females might be due to the effect of the extract on sperm functions. As discussed above, the extract might disable spermatozoa to fertilize by inducing a precocious acrosome reaction, or by causing death or membrane damage. Further meticulous investigations are required possibly by employing in vitro fertilization technique to analyze whether infertility is due to the effect of extract on other functions of the spermatozoa such as capacitation, zona binding/penetration, sperm-oocyte fusion, and oocyte activation. Infertility of the treated females might also be due to the failure of embryo implantation.
The hexane fraction of the two plants Achyranthes aspera and Stephania hernandifolia in combination was found to be an effective spermicidal which caused 100% infertility in rats when applied as a vaginal contraceptive (Paul et al., 2010). However, this herbal formulation was found to be highly toxic and killed all sperm cells in 20 mins by membrane damage (Paul et al., 2005; 2010). In comparison, the Dioscorea extract treatment at 1,000 µg·mL-1 for 2 h resulted in membrane damage in only 20% of spermatozoa.
The purified active principles of herbal extracts have also been studied by some investigators and have produced some promising results. Curcumin from Curcuma longa and triptonide from Tripterygium wilfordii are two such products. Curcumin immobilizes spermatozoa, decreases the acrosome reaction, and reduces the fertility of female mice when applied intravaginally (Naz, 2011). On the other hand, triptonide renders male mice infertile by disrupting spermiogenesis (Chang et al., 2021).
Dioscorea bulbifera (yam) is a widely acclaimed medicinal plant possessing various phytochemicals like polyphenols, tannins, flavonoids, terpenoids, saponin, steroid, and glycoside derivatives including dihydrodioscorine (Liu et al., 2009; Galani and Patel, 2016). An earlier study has shown that oral intake of Dioscorea causes infertility in female rats (Atsukwei et al., 2015). But feeding extract also causes systemic effects leading to the imbalance of sexual hormones (Atsukwei et al., 2015). In the present experiment, we have avoided systemic or long-term exposure to the extract. Intravaginal injection of the extract, performed in our experiments is a topical application. This was sufficient to cause infertility in mice. A previous study has shown that the application of a wild yam (Dioscorea villosa) ointment on the skin is safe and does not affect hormone levels (Komesaroff et al., 2001). It is popularly used to treat menopausal symptoms. Dioscorea possesses a specific saponin diosgenin, a principal material used for the synthesis of corticosteroids and estrogen (Balandrin et al., 1985; Jayachandran et al., 2016). The possibility that diosgenin or its derivatives might be the contraceptive principle in the extract cannot be overlooked (Sautour et al., 2007; Liu et al., 2017). Other well-characterized constituents in the extracts are dioscorine, a potent neurotoxin (Nagata et al., 1999), and dioscorin, an angiotensin converting enzyme (ACE) inhibitory protein (Nagai and Nagashima, 2006) that may affect sperm functions.
Conclusion
Dioscorea bulbifera tuber extract induces an acrosome reaction, viability loss, and membrane damage in mouse spermatozoa. Intravaginal application before copulation causes complete sterility in female mice. These results indicate that the Dioscorea bulbifera tuber extract could be an effective, topically applicable contraceptive for use just before copulation.
Acknowledgements
We are thankful to Prof KD Manandhar, the Chairperson of the Central Department of Biotechnology/Tribhuvan University, Kathmandu, Nepal, for allowing the use of the laboratory facilities for conducting our research. YJ Yi was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (NRF-2020R1A2C1014007).
Authors Information
Jawaswori Sharma, https://orcid.org/0000-0002-5123-7396
Sabina Bhandari, https://orcid.org/0000-0002-6913-9268
Sarbesh Rijal, https://orcid.org/0000-0002-1520-7325
Ramanuj Ranuniyar, https://orcid.org/0000-0002-9672-2686
Young-Joo Yi, https://orcid.org/0000-0002-7167-5123
Gaurishankar Manandhar, https://orcid.org/0000-0002-8145-9475




