Introduction
Lupins is a valuable protein source for pigs and Lupinusangustifolius (L. angustifolius) have been used widely in diets for growing pigs. Cultivars of L. angustifolius contain more protein, fat and energy and thus offer more potential as an ingredient for pig diets. The levels of anti-nutritional factors are the same for both species but are comparatively low and are unlikely to constrain the utilization of these lupin species for animal feeding. However, the extreme bitterness of the seed generally made lupin unsuitable for human and animal consumption, without prior treatment to remove toxic alkaloids, due to presence of anti-nutritional factors including trypsin and chymotrypsin inhibitors (King, 1981). In addition, Knudsen (1997) indicated that the level of total non-starch polysaccharides in lupins is quite higher (405 g·kg-1) relative to other more common sources of protein such as soya-bean meal, peas and canola. The seeds include 20 to 40% protein, which approaches the percentage in soybean meal. The amassment amino acids are similar to that in typical legumes, whereas the content of arginine is higher than that for most other legumes (Yang et al., 2007). The inclusion of lupine seeds had improved growth performance in growing pigs (Kwak et al., 2000) and poultry (Perez-Maldonado et al., 1998; Thamaraikannan and Kim, 2021). Gdala et al. (1996) indicated that dietary supplement with enzymes to increase energy and amino acid digestibility in lupine fed pigs. The use of adequate processing treatments, such as dehulling or heat process, to inactivate or eliminate in most the anti-nutritional factors presents mainly in the hull of grains (Diaz et al., 2006). The supplementation of lupine had negative effects on growth performance in growing pigs (Batterham et al., 1986). Likewise, Petterson (2000) reported that the inclusion levels (20 to 37 %) of lupine had no effects on growth performance in growing pigs. Therefore, the present study was to investigate the effects of different levels of dehulled lupin kernel (DLK) (Lupinusangustifolius) on growth performance, nutrient digestibility, BUN and creatinine, fecal microbiota and fecal noxious gas emission in growing pigs.
Materials and Methods
The Animal Care and Use Committee of Dankook University reviewed and approved the experimental protocols describing the management and care of animals.
A total of 108 growing pigs (Landrace × Yorshire × Duroc) with an average initial body weight (IBW) of 24.49 ± 3.2 kg were allocated in one of three dietary treatments (5, 10, and 20% DLK) according to sex and BW in a randomly complete block design for 6-week. Each dietary treatment consisted of 9 replication pens with 4 pigs per pen (2 gilts and 2 borrows). Diets (Table 1) were formulated to meet or exceed the nutrient requirements recommended by NRC (2012). Pigs were housed in an environmentally controlled, slatted-floor facility with 27 adjacent pens (1.8 m × 1.8 m) at the pig farm of Dankook University. Each pen was provided with a self-feeder and a nipple drinker to allow ad libitum access to feed and water throughout the trial period.
Individual pig body weight was measured initially and finally the trial period, feed consumption was recorded on a pen basis to calculate average daily gain (ADG), average daily feed intake (ADFI) and growth efficiency (G/F). To determine dry matter (DM), nitrogen (N), and energy digestibility, chromium oxide was added to the diet as an indigestible marker at 2 g·kg-1 of the diet for seven days prior to fecal collection. Fecal samples were randomly collected from 2 pigs per pen from 4 pens randomly selected per treatment (n = 8) via rectal massage, and the sample was stored in a freezer at -20℃ until analyzed. Before chemical analysis, fecal samples were dried at 70℃ for 72 h, grounded well, and passed through a 1-mm screen sieve.
Fecal samples were analyzed for DM (method 930.15) and N (method 988.05) following the procedure established by Association of Official Analytical Chemists (AOAC International, 2000). The N (protein) was determined by a Kjeltec 8600 Nitrogen Analyzer (Foss Tecator AB, Hoeganaes, Sweden). Parr 6400 oxygen bomb calorimeter (Parr Instrument Co., Moline, IL, USA) was used to determine the gross energy (GE) by measuring the heat of combustion. A UV/VIS spectrophotometer (Optizen POP, Daejon, Korea) was used to analyze chromium oxide. For calculating the ATTD, the following formula was applied: Digestibility = 1 - [(Nf × Cd)/(Nd × Cf)] × 100, where Nf = nutrient content in feces (% DM), Nd = nutrient content in the diet, Cd = chromium content in the diet and Cf = of chromium content in the feces.
Two pigs (1 barrow and 1 gilt) each pen from randomly selected 4 pens (n = 8) per treatment were bled via jugular venipuncture using a sterile needle. Blood samples from the same pigs per treatment on d 7, 21, and 42 were collected into 5 mL vacuum tubes containing no additive (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ, USA) to obtain serum. Serum was separated by centrifugation at 3,000 × g for 15 min at 4℃. The concentration of blood urea nitrogen (BUN) in the serum was measured with an automatic biochemical analyzer (Model 7020, Hitachi, Tokyo, Japan). The serum creatinine concentration was determined using an Astra-8 analyzer (Beckman Instruments Inc., Brea, CA, USA).
At the end of the trial, the fresh fecal samples were randomly collected from 2 pigs per pen from 4 pens randomly selected per treatment (n = 8) by rectal stimulation to determine ammonia (NH3), hydrogen sulfide (H2S), and mercaptans (R.SH). Then the collected samples were fermented for 24 h at room temperature (25℃). After fermentation period, the samples were shaken manually approximately 30s to disrupt any crust formation and to homogenize. The adhesive plasters were punctured, and 100 mL of headspace air was sampled approximately 2.0 cm above the slurry surface. A gas sampling pump (Model GV-100, Gastec Corp., Ayase, Japan) was utilized for gas detection (Gastec detector tube No. 3La for NH3, No. 4LK for H2S, No. 70 for R.SH, Gastec Corp., Kanagawa, Japan).
All data were analyzed as a randomized complete block design using the PROC MIXED procedure of SAS/STAT®9.4 (SAS Institute Inc., Cary, NC, USA). The each pen was served as the experimental unit. Data on growth performance and nutrient digestibility were based on a pen basis, whereas data on hematology, fecal microbiota were based on individual pig. Orthogonal contrasts were used to the effect of treatments: Control DLK5 vs. others and DLK10 vs. DLK20 treatments. Variability in the data was expressed as standard errors of mean (SEM). A probability level of less than 0.05 was considered as statistically significant.
Results
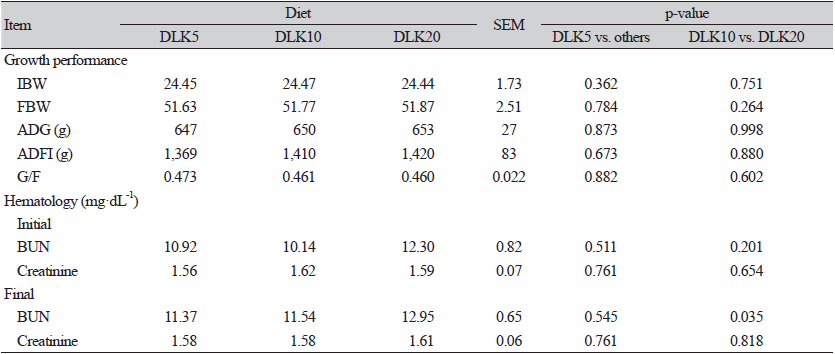
No significant effects were observed in ADG, ADFI, G/F, and final body weight (FBW) among the treatments (p > 0.05; Table 2). However, pigs fed the DLK20 diet had higher serum BUN compared with those fed the DLK10 diet (p < 0.05). There were no significant differences among treatments in nutrient digestibility (p > 0.05) measured in growing pigs (data not shown). There were also no significant differences (p <0.05) among treatments in fecal microflora and fecal noxious gas emission (data not shown).
Discussion
In the current study showed that pigs fed DLK had no significant effects on growth performance and nutrient digestibility. Our results are consistent with the study of Yang et al. (2007) who reported that diets supplemented with DLK had no significant effect growth performance and nutrient digestibility in growing pigs. Similarly, Gdala et al. (1996) and Mullan (2001) noted that dietary supplementation of lupine had no effects on growth performance in growing and finishing pigs. Kwak et al. (2000) who reported that dehulled lupin kernel supplement had no differences in nutrient digestibility in growing-finishing pigs. In contract, Fernandez and Batterham (1995) and Flis et al. (1996) reported that dietary inclusion of DLK could improve growth performance and nutrient digestibility in growing pigs. Feeding lupine to pigs was interrelated with decreased feed intake, owing to extended retention time in the stomach (Dunshea et al., 2001). The dietary inclusion of 15% lupine had significantly reduced feed intake and daily gain in growing pigs (Van Nevel et al., 2000) and reduced nitrogen retention in young pigs (Gdala et al., 1996). The inconsistent result on growth performance and nutrient digestibility among different studies could be due to diet complexity, inclusion level of DLK, sex or age and the health status of the pigs and other environmental or management factors.
The BUN concentration and production are influenced by protein catabolism, and its concentration is negatively correlated with the utilization of proteins and AA (Coma et al., 1995). Creatinine is a natural waste product created by the muscles and is removed from the body by the kidney. In the current study, DLK20 diet had higher serum BUN level compared with the DLK10 diet. It is suggested that BUN can be conversely related to the efficiency of protein and protein quality (Coma et al., 1995), though the serum BUN level in the current study in DLK20 diet (12.95 mg·dL-1) is within the normal range (Devi et al., 2014) compared to the DLK10 diet (11.54 mg·dL-1). Lupine has a high content of protein which may another possible reason to increase the BUN during the experimental (Hove, 1974). Gut health is a one of the major factor governing the performance of pigs and thus, the economics of swine production (Kim et al., 2007) while the profile of intestinal microflora plays an important role in gut health. The dietary dehulled lupine kernel is able to impair microbial growth in the food and consequently to preserve the microbial balance in the gastrointestinal tract. In the present study, no significant difference in fecal microbial among the treatments. The fecal microbial-based on dehulled lupine kernel supplementation in growing pigs has not been reported; thus, comparisons could not be made.
As we know, fecal noxious gas emission such as NH3and H2S has become one of the major air pollutions in modern concentrative swine production (Zhang et al., 2013). High concentrations of H2S or NH3 can cause hazard impacts to humans and animals (Drummond et al., 1980, Zhang and Kim, 2013). It has been suggested that fecal noxious gas emission is related to the gut bacterial ecological system and nutrient utilization (Ferket et al., 2002). In the present study, fecal gas emission was not affected by dietary treatments. The reason may be due to the insignificant differences were observed in nutrient digestibility and fecal microbiota among the treatments. As, DLK contained a considerable proportion of dietary fiber (Guillon and Champ, 2002), could promotes E. coli and Lactobacillus sp. (Wang et al., 2009), thus, the effects of dehulled lupin kernel on fecal microbial shedding and fecal noxious gas emission in growing pigs need further research.