Introduction
Beekeeping by-products are produced by bees and their hives, and they usually contribute positively to health maintenance, well-being, and reductions in chronic diseases (Pasupuleti et al., 2017). There are a variety of beekeeping by-products, including propolis, royal jelly, and bee venom, and functional studies for their use in the livestock industry are underway.
Propolis (PRO) is a resinous substance accumulated by bees from various plants and contains active substances such as polyphenols and terpenoids (Babaei et al., 2016; Pasupuleti et al., 2017). The principal active substances in PRO have been reported to have antibacterial, antiviral, antifungal, antioxidant, and anti-inflammatory effects in animals (Al-Nayef and Al-Nuaimi, 2015; Haščík et al., 2015). Royal jelly (RJ), a white and viscous substance, is secreted by the hypopharyngeal and mandibular glands of worker bees and is a special nutrient used for feeding larvae and queen bees (Strant et al., 2019). RJ contains mainly proteins with high levels of essential amino acids and peptides with anti-inflammatory, immunomodulatory, and antioxidant properties (Okamoto et al., 2003; Pavel et al., 2011). The beehive is a natural resinous bee by-product and contains many residues of bee products such as beeswax, cocoon, and honey (Song et al., 2022). Active ingredients such as flavonoids, polysaccharides, and polyphenols in beehives have been reported to have anti-cancer, anti-inflammatory, and bactericidal effects (Choi and Nam, 2020; Nader et al., 2021).
Previous studies reported that the addition of beekeeping by-products to animal diets had a positive effect on animal growth performance and internal function (Rabie et al., 2018; Seven et al., 2014). However, few studies have confirmed the effect of adding beekeeping by-products to drinking water. Therefore, this study was conducted to evaluate the effect of adding beekeeping by-products to drinking water on the growth performance, and intestinal and fecal microflora of Institute of Cancer Research (ICR) mice.
Materials and Methods
The protocol for this study was reviewed and approved by the Institutional Animal Care and Use Committee of Chungbuk National University, Cheongju, Korea (CBNUA-1739-22-01).
Preparation of beekeeping by-products
The PRO and RJ used in this study were obtained by a commercial company (Ohfarm, Seoul, Korea). The beehive extract (BE) was used after extracting the beehive in 50% ethanol and concentrated for a long period of time.
Animals and experimental design
A total of 72 five-week-old ICR mice with an initial body weight (BW) of 24.57 ± 0.60 g were used in a two-week experiment. The ICR mice used in this study were purchased from the DBL (Eumseong, Korea). All ICR mice were assigned to a completely randomized into four treatment groups based on the initial BW. There were 3 mice in a cage and six replicate cages per treatment. The treatment groups were as follows: 1) CON, normal distilled water (DW); 2) T1, CON with 0.7% BE; 3) T2, CON with 0.7% PRO; 4) T3, CON with 0.7% RJ. The mice were housed in a temperature and humidity-controlled room with a 12 : 12 h light-dark cycle. Commercial diets and experimental drinking water were provided ad libitum for the experimental period.
Sampling and measurements
Growth performance
BW was measured for an individual at the experiment’s beginning, 1-week, and final (2-week). Feed intake (FI) and drinking water intake were recorded daily the supply amount and the remaining amount. Feed efficiency (G : F) was calculated by dividing the body weight gain (BWG) by the FI. BWG, FI, G : F, and drinking water intake were calculated for each interval from 0 - 1 weeks, 1 - 2 weeks, and 0 - 2 weeks.
Microbiological analyses
At the end of the experiment, all mice were sacrificed by cervical dislocation. Fresh feces were collected for microbiological analysis, and large intestinal contents were also collected after cervical dislocation. One gram of each fecal and large intestinal content sample was diluted with 9 mL of aseptic distilled water and homogenized. Samples were used for measuring the number of viable cells by serial dilution from 10-2 to 10-6. Bacterial colonies were counted by the pour plate method. To measure the number of colonies of Escherichia coli (E. coli), Salmonella, and Lactobacillus, MacConkey agar was used for E. coli, BG Sulfa agar for Salmonella, and de Man, Rogosa and Sharpe (MRS) agar for Lactobacillus. All agars used for the analysis were purchased from KisanBio (Seoul, Korea). E. coli and Salmonella were cultured at 37℃ for 24 hours. Also, Lactobacillus was cultured at 37℃ for 48 hours. The number of E. coli, Salmonella, and Lactobacillus colonies was counted immediately after plates were removed from the incubator. The number of the microbial colonies was log-transformed before statistical analysis.
Statistical analysis
All data were statistically processed using the general linear model procedures of SAS (SAS Institute, Cary, NC, USA). Differences between treatment means were determined using Tukey’s multiple range test. A probability level of p < 0.05 was indicated to be statistically significant, and a level of 0.05 ≤ p < 0.10 was considered to have such a tendency.
Results
Growth performance
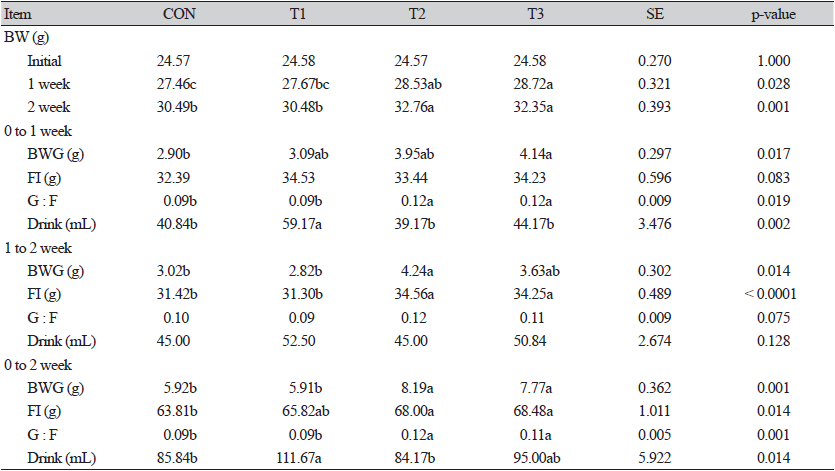
There was no difference between treatment groups in the initial BW of mice (Table 1). In weeks 1 and 2, T2 and T3 showed significantly higher (p < 0.05) BW than CON. At 0 - 1 week, T3 showed significantly higher (p <0.05) BWG and G : F than CON. Compared with CON, T2 showed significantly increased (p <0.05) BWG and FI at 1 - 2 weeks. During the entire period (0 - 2 weeks), T2 and T3 showed significantly higher (p <0.05) BWG and G : F than CON and T1. In the drinking water intake from 0 - 1 week, T1 was significantly higher (p < 0.05) than that of other treatment groups. There was no significant difference (p > 0.05) in drinking water intake between the treatment groups at 1 - 2 weeks. During the entire period, T1 showed a significantly increased (p < 0.05) drinking water intake compared to CON.
Intestinal and fecal microflora
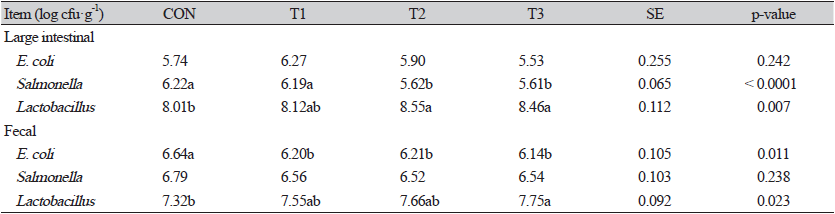
The amount of Salmonella in the large intestinal at week 2 was significantly lower (p < 0.05) in T2 and T3 than in CON and T1 (Table 2). In Lactobacillus amounts of large intestinal, T2 and T3 showed significantly higher (p < 0.05) values than CON. The amount of E. coli in the fecal was significantly reduced (p < 0.05) compared to CON in all the treatment groups to which beekeeping by-products were added to drinking water. The amount of Lactobacillus in the fecal was significantly higher (p < 0.05) in T3 than in CON.
Discussion
The results of our study showed that the addition of PRO or RJ to drinking water improved the growth performance of mice. Over the entire period, the addition of PRO increased the BWG of mice by 38% and improved feed efficiency by 33%. The addition of RJ increased the BWG of mice by 31% and improved feed efficiency by 22%. In a previous study, Babaei et al. (2016) reported that the addition of PRO or RJ to the quail diet improved BWG and the feed conversion ratio. It was also reported that adding PRO increased FI in broilers and quail (Seven et al., 2012; Fouad, 2021). In this study and previous studies, the increase in FI with the addition of PRO seemed to be due to the increase in palatability caused by the flavonoids contained in PRO (Seven et al., 2012). PRO contains vitamins, flavonoids, and minerals, which are important substances that improve the growth performance of animals, and RJ contains many flavonoids, organic compounds, and fatty acids (Pasupuleti et al., 2017; Mohamed et al., 2018). In particular, the flavonoids contained in PRO and RJ could reduce pathogenic bacteria and improve digestibility by stabilizing intestinal flora (Soltani et al., 2019). Thus, this may have been the reason feed efficiency was improved by the addition of PRO or RJ in this study.
In this study, drinking water intake was higher in the treatment group with BE added to the water than in the control group with nothing added to the water. According to Song et al. (2022), BE has an aromatic odor and a stimulating effect on the salivary and gastric glands, which may increase feed or drinking water intake. Therefore, it seems that drinking water intake increased because of the increase in palatability due to BE in this study as well. However, there are few studies on adding BE to drinking water in mice, so additional study on the effect of BE on drinking water intake is needed.
PRO or RJ positively modulated large intestinal and fecal microflora in this study. Aabed et al. (2019) reported that supplementation with bee pollen and PRO to mice helped to stabilize the gut microbiota. Flavonoids such as pinocembrin and naringenin contained in PRO exhibited antibacterial effects against gram-positive and gram-negative bacteria and also exerted antiviral activity (Temiz et al., 2011; Cornara et al., 2017). In this study, the beneficial effects of the biologically active materials in PRO may have contributed to decreasing harmful bacteria such as E. coli and Salmonella in the intestine and increasing Lactobacillus, beneficial bacteria. A previous study reported that the administration of RJ to mice reduced the relative abundance of Alistipes in the intestine, helping to modulate the intestinal microflora and enhance antioxidant activity (Chi et al., 2021). The main physiologically active compounds of RJ, such as proteins, peptides, lipids, phenols, and flavonoids, have been reported to regulate intestinal microflora and enhance antioxidant activity (Kocot et al., 2018). Also, RJ showed certain antibacterial activity, and the major royal jelly proteins 2-5 (MRJP 2-5) were reported to exert antibacterial activity, especially against gram-negative E. coli (Park et al., 2019). Therefore, the mechanism by which RJ decreased the amount of fecal E. coli and increased the amount of Lactobacillus in our study might be due to inhibiting the growth of gram-negative bacteria through the antibacterial action of RJ and regulating the intestinal microflora.
Conclusion
During the entire period, the addition of PRO or RJ to drinking water improved the BWG, FI, and feed efficiency of mice. Also, in the microflora in the intestine and feces, PRO and RJ showed a decrease in the amount of E. coli and an increase in the amount of Lactobacillus compared to mice fed with normal drinking water, and it seems that they had a positive effect on the intestinal microflora. In conclusion, the addition of PRO or RJ to the drinking water of ICR mice had a positive effect on growth performance and intestinal and fecal microflora.
Acknowledgements
This research was supported by "Regional Innovation Strategy (RIS)" through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (MOE) (2021RIS-001).
Authors Information
Se Yeon Chang, https://orcid.org/0000-0002-5238-2982
Ji Hwan Lee, https://orcid.org/0000-0001-8161-4853
Han Jin Oh, https://orcid.org/0000-0002-3396-483X
Yong Ju Kim, https://orcid.org/0000-0002-0960-0884
Jae Woo An, https://orcid.org/0000-0002-5602-5499
Young Bin Go, https://orcid.org/0000-0002-5351-6970
Dong Cheol Song, https://orcid.org/0000-0002-5704-603X
Hyun Ah Cho, https://orcid.org/0000-0003-3469-6715
Yun A Kim, https://orcid.org/0000-0002-5505-030X
Sang Hun Park, https://orcid.org/0000-0003-4804-0848
Yun Hwan Park, https://orcid.org/0000-0002-2239-6697
Gyu Tae Park, https://orcid.org/0000-0003-1614-1097
Se Hyuk Oh, https://orcid.org/0000-0003-4105-2512
Jung Seok Choi, https://orcid.org/0000-0001-8033-0410
Jin Ho Cho, https://orcid.org/0000-0001-7151-0778