Introduction
Recently, plant extracts especially medical plants, replacing antibiotics as growth promoters in the animal industry have been reported (Astorga and Martín, 2014; Mohankumar et al., 2021). Red ginseng is made from ginseng that undergoes steam boiling and drying, extending shelf life. Red ginseng extract as a by-product of processing red ginseng consists of fiber, polysaccharides, flavonoids, and saponin, and could as a potential and utilizable resource in the breeding industry. To date, evidence shows that red ginseng extract (RGE) could be effective in enhancing immune functions (Lu et al., 2009), relieving fatigue (Bach et al., 2016), anti-cancer and antioxidant (Seo and Kim, 2011) in humans, and has more content of Rg3 and Rh2 in comparison with untreated red ginseng, which was effective for anti-cancer (Wang et al., 2020). Yin et al. (2018) revealed that supplementation of 0.4% RGE in the diet improved the nitrogen (N) and dry matter (DM) digestibility of weaning piglets.
On the other hand, fermented red ginseng (FRG) extract is obtained by fermentation of freeze-dried red ginseng marc using Bacillus subtilis and Bacillus coagulans. Due to an increase in polyphenol and ginsenoside contents, and higher activity of saponin after microbial fermentation, FRG shows stronger antioxidant properties compared with unfermented red ginseng (Ryu et al., 2013) and is easily absorbed by the gastrointestinal tract. Besides, FRG possesses a better flavor and is enriched with microbial metabolites (Peres et al., 2012). Given that FRG supplementation to diets improved the dry matter (DM) digestibility and reduced the counts of fecal Escherichia coli in weaning pigs (Yin et al., 2018), whereas 0.2% FRG supplementation to fattening diets declined the average daily feed intake (ADFI) during weeks 1 to 4, and 0.4% FRG in diets declined ADFI over weeks 5 to 8 but decreased drip loss and water holding capacity (WHC) in fattening pigs (Ao et al., 2011a). In broilers, 0.1% and 0.2% dietary supplements of FRG improved the meat color on day 30 (Ao et al., 2011b).
There are limited studies on the supplementation of FRG or RGE to the diet of growing-fattening pigs. The study aimed at evaluating the impact of FRG and RGE on the growth, nutrient digestibility, and meat quality of growing-fattening pigs.
Materials and methods
These pigs were raised in the swine experiment base of Dankook University in Cheonan city (Republic of South Korea). Procedures implemented on animals and sampling collection abided by the Animal Care and Use Committee of Dankook University (DK-1-1712).
Experiment design
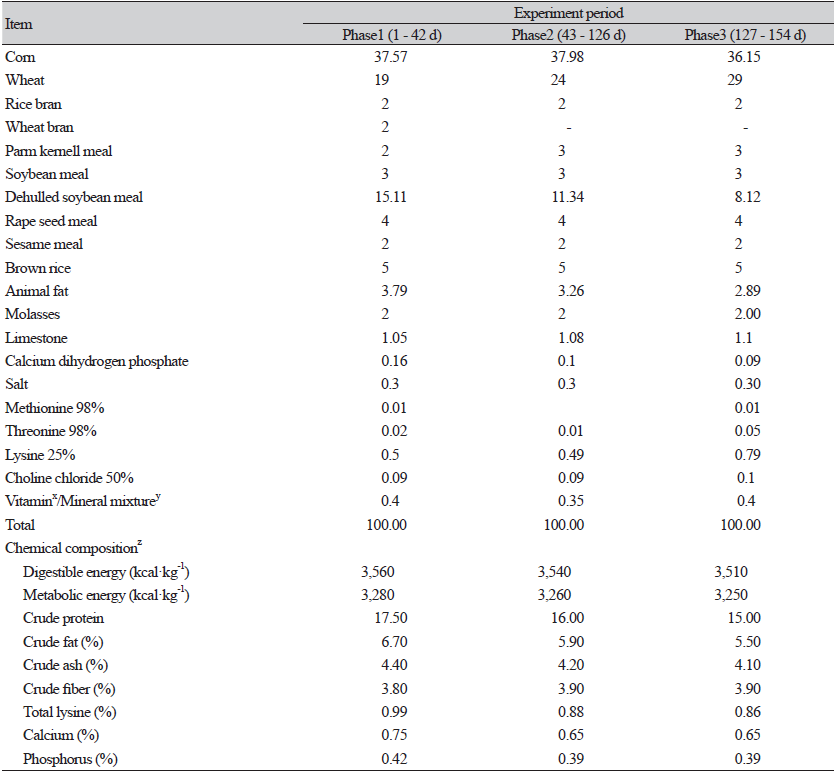
In total, 120 growing pigs (landrace × [Yorkshire× Duroc]) with average body weight (BW) of 21.77 kg (SE1.88) were raised for a 22-week trial. Pigs were randomly allocated into 3 treatments with 8 repeat pens (3 gilts and 2 barrows per pen) based on BW using a completely randomized design. The pigs were provided either basal diet (CON group) or the basal diet supplemented with 1 g·kg-1 FRG or 1 g·kg-1 RGE. The basal diets (Table 1) were maize-soybean meal diets for 3 rearing phases (1 - 42, 43 - 126, 127 - 154 days) and met the recommendations of NRC (2012). Pigs had free access to feed and water. The ambient temperature was controlled at 22℃, and ventilation was regulated by mechanical devices.
Preparation of the additives
Fermented red ginseng and red ginseng extract were purchased from Sunbio Medical Co., Ltd. (Gunpo, Korea). FRG was obtained by inoculating a mixture of 300 g sterilized freeze-dried red ginseng marc and 1 L water as the substrate with 0.1% (w·w-1) microorganisms (Bacillus subtilis and Bacillus coagulans) for 24 h at 700 × g, at 30℃.
RGE was made by steaming fresh ginseng and freeze-drying, including ginsenoside monomer Rb1, 7.44 mg·g-1; Rb2, 2.59 mg·g-1; Rc, 3.04 mg·g-1; Rd, 0.91 mg·g-1; Re, 1.86 mg·g-1; Rf, 1.24 mg·g-1; Rg1, 1.79 mg·g-1; Rg2, 1.24 mg·g-1; Rg3, 1.39 mg·g-1; Rh1, 1.01 mg·g-1, and other minor ginsenosides.
Sample collection and measurement
Individual BW of the fasted pig was weighed on the first day and at the termination of weeks 6, 10, 18, and 22 in the morning. Feed consumption was recorded at the termination of weeks 6, 10, 18, and 22 regarding per pen as an experimental unit. Growth performance parameters were calculated containing average daily gain (ADG), ADFI, and gain: feed ratio (G : F).
To assay the digestion and absorption of nutrients, pigs were supplemented with the diet containing 2 g·kg-1 chromium oxide during weeks 6,10, and 18. Fecal samples were collected (1 male and 1 female) from randomly selected 5 pens per treatment at last three days of these periods by stimulating the pig's anal sphincter to induce defecation, and 10% dilute sulfuric acid and toluene (Duksan Techopia Co., Ltd., Cheonan, Korea) were used to fix the manure. These three days’ feces were mixed and pooled in equal proportions, then refrigerated at -20℃ prior to analysis.
Frozen feedstuff and feces were thawed and weighed before being put into an electric oven (Daihan Scientific Co., Ltd., Seoul, Korea) and heated to 72℃ for up to 60 h until constant weight, and it was then ground through a 40-mesh screen. Parallel content determination was carried out for individual samples. DM in feed and feces samples was assayed with method 930.15 (AOAC, 2000). N levels were determined by using the principle of Method 920.40 (AOAC, 2000), with KjeltecTM8400 instrumentation (FOSS, Copenhagen, Denmark). For gross energy measurement, caloric content generated from the burning of feed and fecal was measured via a Parr 6400 calorimeter (Parr Instrument Co., Moline, IL, USA). The concentrations ofCr2O3 were recorded based on the method reported by Lei and Kim (2014). The calculation of apparent total tract digestibility (ATTD) was carried out as follows: digestibility, (%) = [1 − {(Nf × Cr2O3d)∕(Nd × Cr2O3f)}] × 100, where Nf and Nd represented nutrient concentration, andCr2O3f andCr2O3d represented chromium concentration, each in feces and diet, respectively. These values were all presented as percentages of the total dry matter.
Blood samples (10 mL) were collected using K3EDTA evacuated tubes (3.0 mL; Greiner Bio-One, Kramsmenster, Austria) by puncturing the anterior vena cava from 5 fasted piglets for each treatment at the end of weeks 6, 10, and 18. The serum was obtained after the blood sample was centrifuged (3,500 × g for 15 min, 4℃), and kept at -20℃ prior to analysis. The concentrations of blood urea nitrogen, creatinine, triglycerides, and cholesterol in serum samples were determined with a HITACHI 7180 automatic biochemical analyzer (Hitachi, Tokyo, Japan). The activity of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) was determined using ELISA kits (Catalogue NO: abx360984, abx575766; Abbexa, Houston, USA) and a microplate reader (SpectraMax190, Molecular Devices Corporation, California, USA) at wavelengths of 510-nm and 505-nm. The coefficient of variation between inter- and intra- assay ranged from 10% (intra-assay) to 15% (inter-assay).
At the termination of the 22-week trial, 120 pigs were slaughtered at a local slaughterhouse. Pigs went through electric shock, bleeding, depilation, and removal of viscera (preserving kidneys and belly fat). Afterward, hot carcass weight (HCW) weighed individually was used for calculating the slaughter rate ([live weight-HCW]/live weight). Precisely, the left side longissimus thoracic (LT) in terms of meat quality were cut between the 10th and 11th ribs from randomly selected 6 pigs for each treatment and stored at 4℃ in cold storage for 24 h. To assess the effect of dietary supplements on antioxidant properties, 20 g of LT were frozen at -20℃ for further analysis.
The loin-eye area was measured by firstly painting the profile of the cross-section of LT at the 10th rib and then calculating the profile with a planimeter. The pH measurement was implemented at 0, 6, 12, and 24 h postmortem resorting to a pH meter (OPTO-STAR, R. Matthaus, Germany) with parallel probes in different positions of the meat samples. The probe of the pH meter was calibrated with standard buffers of 4.86 and 7.0. The marbling and firming scores were evaluated by 2 professional staff referring to the National Pork Producer Council (NPPC, 1999) 24 h after slaughter. The L* (lightness), a* (redness), and b* (yellowness) values for scoring the meat color were taken into account and obtained from the average of 3 random readings via Opto-star flesh color tester (R. Matthaus, Germany), respectively at posthumous 45 min. The drip loss was evaluated with the formulation: the weight loss after suspension/ initial diced meat weight. In brief, individual diced meat (40 ± 5 g) with a length and width of 3 cm was cut from LT and then put into a sealed sample bag in triplicate. The sample bags were hung at 4℃ for 24, 72, and 120 h before reweighing. The ratio of water: meat area was then calculated, giving a measure of WHC (a smaller ratio indicates increased WHC). For assay of cooking loss, approximately 100 g fresh muscle samples were steamed for 40 min in duplication, after which the samples were removed from the steamer, cooled, and reweighted.
Before the measurement of oxidative properties in the meat sample, the frozen muscle sample at 24 h postmortem was ground into powder in a sterile mortar together with liquid nitrogen. Then 1 g powder abrasive mixed with 9 mL 9% saline (at 4℃) was centrifuged at 3,000 × g, 4℃ for 10 min to get supernatant for assaying oxidative properties. The 2-thiobarbituric acid-reactive substances (TBARS) were detected according to the TBARS kit instructions in triplicate. (Catalogue No: abx097981, Abbexa, Houston, USA). The final values of TBARS were presented using milligrams of malonaldehyde per kilogram of muscle.
Data analysis
The analysis for all data was operated by one-way ANOVA using GLM procedure of SAS 9.4 (2000, SAS Inst. Inc., Cary, NC, USA) with Duncan's multiple comparisons. ADG, feed intake, G/F, HCW, and ATTD were analyzed using pens as experimental units, other indices regarded individual pigs as analysis units. A probability of p ≤ 0.05 was viewed as significant, and a tendency of 0.05 < p ≤ 0.1 was acknowledged.
Results and Discussion
Growth performance and ATTD
Ginseng is well known as a traditional medical herb for improving immunity, curing cancer, and reducing stress because of functional plant polysaccharides and saponins. To date, several researchers reported that fermented ginseng culture could be a potential additive to improve animal growth performance (Van Doan et al., 2019).
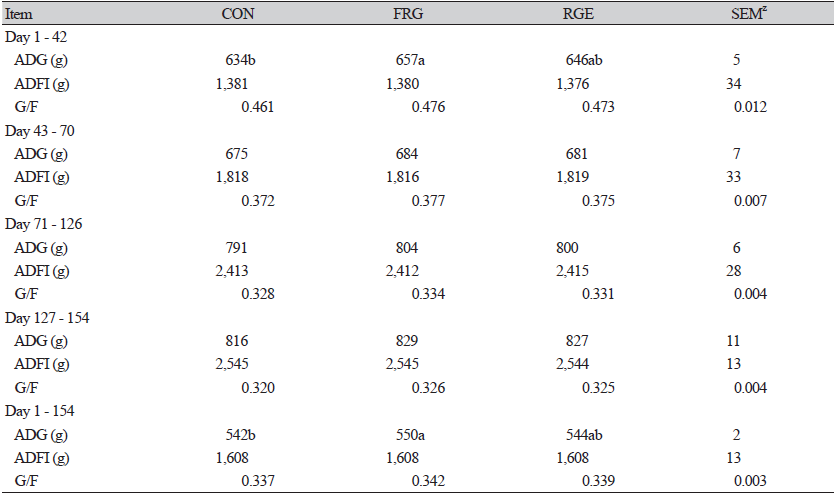
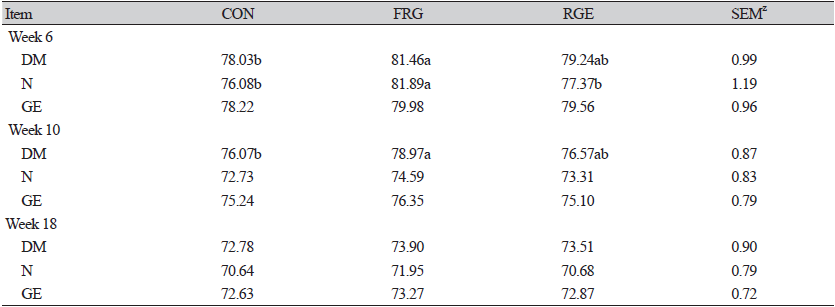
The result of the current study (Table 2) illustrated that pigs fed basal diet containing 0.1% FRG had higher (p<0.05) ADG during days 1 to 42 and the overall experimental period. In contrast to our results, a study by Ao et al. (2011a) showed that a basal diet containing 0.1, 0.2, and 0.4% FRG had no response to growth parameters but reduced ADFI in fattening pigs. In the weaning diet, 0.4% FRG also did not affect the growth of weaning pigs. The difference in growth parameters may be due to the digestive system capacity of different pigs at different ages. Earlier research noted that the improvement of ATTD contributed to the greater growth performance of weaning piglets or fattening pigs (Yoon et al., 2010; Zhao et al., 2013). Whereas in this study ADFI was not different among groups (p > 0.05), the higher digestibility of DM and N (p < 0.05) in FRG pigs may be the possible explanation for higher ADG (Yan et al., 2010) during week 6 of the experiment, as shown in Table 3. It was well known that RGE is mainly composed of carbohydrates and ginsenosides. After bacterial fermentation, ginsenosides are metabolized into biologically active forms, which are easily absorbed by intestinal epithelial cells (Ryu et al., 2013). Contemporary, Ryu et al. (2013) proved that fermented-RGE has a faster nutrient transfer speed than unfermented. Probably the improved nutrient digestibility is due to the active ingredients of fermented red ginseng.
Whereas, no significance (p > 0.05) was observed on growth performance or ATTD when pigs were supplemented with 0.1% RGE in the diet. Inconsistent with the results of Yin et al. (2018), using 0.2% RGE in feed improved the N digestibility of weaning piglets. We guessed the improved immune function and healthier digestive system as pigs grow up weakened the effect of ginseng extract on piglets partially. It also reflected that fermented ginseng extract was more beneficial to the growth of growing pigs than unfermented ginseng extract.
Blood profile
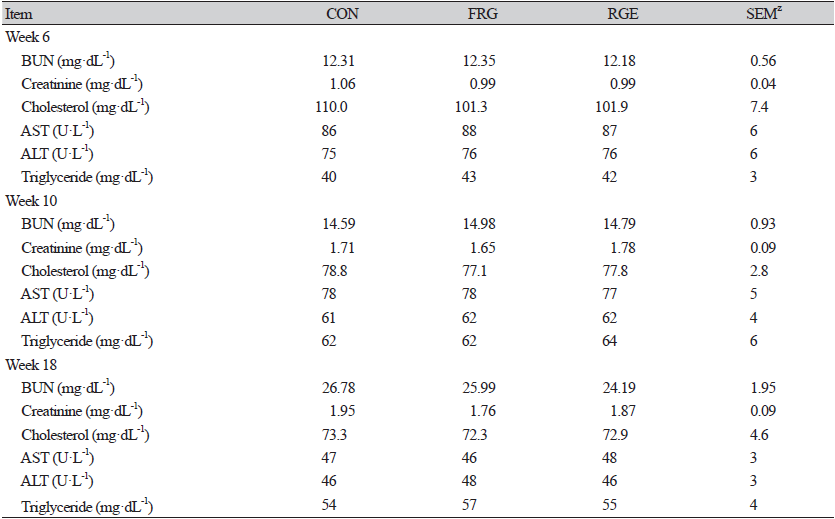
Earlier studies reported that red ginseng had a positive impact on blood profiles (Suh et al., 2002). Kim (2015) showed RGE reduced the levels of cholesterol and triglycerides since ginsenoside combined with cholesterol, which hindered the intestinal absorption of fat (Rao and Gurfinkel, 2000). But in the present study (Table 4), triglyceride or cholesterol concentrations were not different (p > 0.05) when pigs were fed with FRG or RGE diets. The analogous result was obtained in the study of Yin et al. (2018), supplementation of 0.4% RGE to weaning piglets, and Ao et al. (2011a) with the application of dietary 0.1, 0.2, 0.4% FRG on fattening pigs. Moreover, the concentrations of BUN and creatinine could be indicators for evaluating renal function (Dang et al., 2021). Also, elevated serum AST and ALT activity is considered to damage liver cells (Onmaz et al., 2017), and AST activity acts as a representative sign of fat liver (Schiavone et al., 2007). But there was no treatment effect (p > 0.05) on BUN, creatinine, AST, and ALT. Probably it mirrored supplementary 0.1% FRG and 0.1% RGE showed no adverse effects on the renal function of growing-fattening pigs because flavonoids in red ginseng could ameliorate renal damage (Liu et al., 2011).
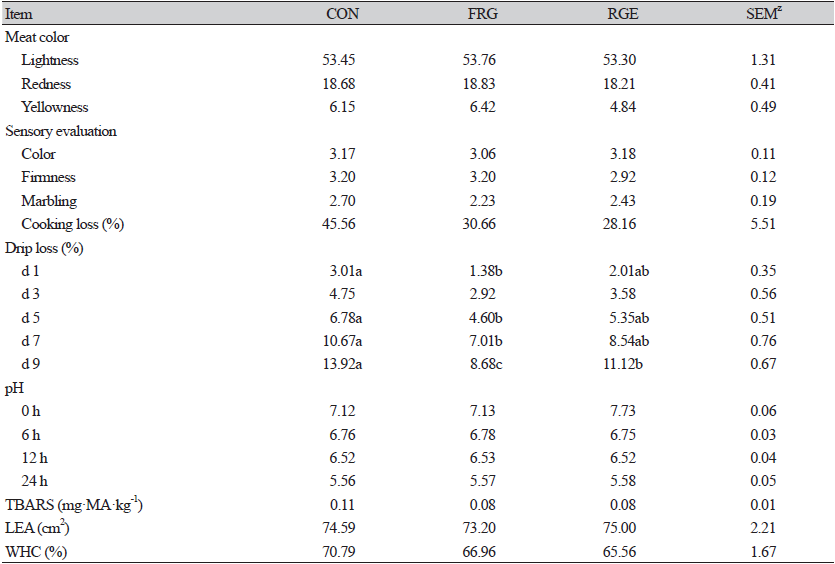
Meat quality
In the case of high-density intensive farming, the growth velocity of fattening pigs and their meat quality have been the principal attention, respectively for raisers, sellers, pundits, and consumers (Valaitienë et al., 2017; Mohankumar et al., 2021). Since high-quality pork with higher nutritional value, excellent flavor, and meat color has won high favor by consumers (Yu et al., 2019).
In Table 5, drip loss in FRG and RGE groups showed a significant reduction (p < 0.05) compared with the CON group. Forrest et al. (2000) showed drip loss could directly reflect better muscle hydraulic power, so the addition of FRG or RGE may be beneficial for better meat quality. While WHC was not affected by dietary 0.1% FRG or 0.1% RGE, which differed from the study of Ao et al. (2011a), who found 0.2% FRG addition improved WHC and reduced drip loss of fattening pigs. WHC was not only related to meat flavor but also affected economic value. Zhu et al. (2019) found that WHC was related to the antioxidant property of meat as increased antioxidant capacity of meat could maintain cell integrity to hinder water loss. Whereas no treatment effects (p > 0.05) on TBARS concentrations were shown in the current study, which may explain the similar WHC.