Introduction
Fertilization is a complex multistep process comprising sperm-oocyte interaction, fusion, and initiation of embryo development (Yanagimachi and Chang, 1963). In vitro fertilization (IVF) is a well-known technique where an egg is combined with sperm In vitro for producing offspring. The efficiency of IVF can be increased by optimizing the techniques involved in the process (Rahman et al., 2020). The IVF media, the viability and number of motile sperm, acrosomal remodeling for capacitation, the sperm-oocyte co-incubation time, and stable embryonic development are important parameters for successful IVF (Sakkas et al., 2001). Spermatozoa must effectively approach and penetrate the ovulated oocyte to complete fertilization. Therefore, in specific conditions like asthenospermia or oligospermia, an auxiliary fuel is needed for the spermatozoa to successfully fertilize the egg (Biswas et al., 2010). The IVF medium plays an important role in promoting efficient fertilization. Several research studies have focused on modifying IVF media formulations to provide more favorable conditions for IVF (Rahman et al., 2020).
Shilajit is a blackish-brown colored exudation found in the Himalayan rock regions of the Indian subcontinent. It is made up of 60 - 80% humus, as well as additional components such as benzoic acid, fatty acid, resin, sterol, amino acids, and phenolic lipids (Agarwal et al., 2007).
Shilajit has been shown to improve reproductive indices including motility, sperm production, serum testosterone, and seminiferous tubule function in cadmium-induced infertile mice (Gupta et al., 2013). In human study, shilajit capsules were taken twice a day for 90 days by male patients with oligospermia, and the sperm count, sperm vitality, and male hormone levels increased in about 47% of patients (Biswas et al., 2010). Additionally, studies also suggest that shilajit has significant anti-inflammatory activity, free radical scavenging action, and anxiolytic effects (Jaiswal and Bhattacharya, 1992; Bhattacharya et al., 1995).
Although Shilajit is effective for reproductive disorders in animals and humans, there are no reports describing the effect of shilajit as a supplement to the IVF medium so far. Therefore, the present study was conducted to evaluate the effects of shilajit on sperm used for the IVF of pig oocytes.
Materials and Methods
Liquid boar semen processing
Liquid boar semen with more than 80% sperm motility was procured from a local artificial insemination (AI) center and used for the current experiment. Sperm concentration was estimated with a hemocytometer and the semen was diluted with Beltsville thawing solution (BTS; Pursel and Johnson, 1976) to a final concentration of 1 × 108 spermatozoa·mL-1. The diluted semen was stored in a storage unit at 17℃ for 5 days.
Unless otherwise mentioned, all other reagents used in this study were purchased from Sigma-Aldrich Chemical Co., LLC (St. Louis, MO, USA).
Preparation of shilajit extracts
Purified shilajit (Gorkha Ayurved Co., Pvt., Ltd., Kathmandu, Nepal) was mixed with phosphate-buffered saline (PBS) solution, vortexed for 10 min, filtered through a 0.45 µm pore size syringe filter, and then used for sperm incubation and IVF.
Assessment of sperm motility
Sperm motility was examined using a computer-assisted sperm analysis system (Sperm Class Analyzer®, Microptic, Barcelona, Spain). Spermatozoa were incubated in the presence of shilajit (final concentration: 0 - 100 µg·mL-1) for 30 min at 37.5℃. Then a 2 μL aliquot of sperm sample was placed in a pre-warmed (380℃) Leja counting slide (Leja products B.V., Nieuw-Vennep, The Netherlands), and 10 fields were analyzed at 37.5℃, assessing a minimum of 1,000 spermatozoa per sample. The proportion of total motile spermatozoa (%), progressive motile spermatozoa (%) and hyperactive spermatozoa (%) were determined. The kinetic parameters measured for each spermatozoon included the curvilinear velocity (VCL, μm·s-1), the straight-line velocity (VSL, μm·s-1), the average path velocity (VAP, μm·s-1), the percentage of linearity (LIN, %), the percentage of straightness (STR, %) and the wobble (WOB, %).
Measurement of acrosomal integrity of spermatozoa
Boar spermatozoa (1 × 107 spermatozoa·mL-1) were incubated in BTS with or without varying the concentration of shilajit for 30 min at 37.5℃. For the assessment of acrosomal integrity, the spermatozoa were stained with 10 μg·mL-1 lectin peanut agglutinin-fluorescein isothiocyanate (PNA-FITC) conjugate and propidium iodide (PI). Then images were acquired on a fluorescence microscope (Nikon Eclipse Ci microscope, Nikon Instruments Inc., Seoul, Korea) with a camera (DS-Fi2, Nikon) and imaging software (version 4.30, Nikon). The spermatozoa were classified as intact (PNA+) or damaged (PNA-) acrosomal sperm, and live (PI-) or dead (PI+) sperm.
Measurement of adenosine triphosphate (ATP) content in spermatozoa
Sperm incubated with shilajit were washed twice with PBS, collected by centrifugation at 800 × g for 5 min, and adjusted to a concentration of 2 × 106 sperm·mL-1. The sperm ATP content was determined using a luciferase reaction kit according to the manufacturer’s protocol (ATPliteTM, Perkin Elmer Inc., Boston, MA, USA). Standards were prepared from an ATP standard (Perkin Elmer) using serial dilutions to obtain concentrations of 1 × 10-7, 5 × 10-8, 1 × 10-8, 5 × 10-9, 1 × 10-9, 5 × 10-10, and 1 × 10-10 M. Aliquots of the ATP stock solution were stored at -20℃ until use, and standard curve dilutions were prepared for each assay. Bioluminescence was measured with a high throughput screening (HTS) multi-label reader (Perkin Elmer) after the addition of a 100 µL sample and 100 µL luciferin-luciferase reagent (Yi et al., 2009).
Assessment of intracellular reactive oxygen species (ROS) and cytoplasmic Ca2+ level in spermatozoa
Boar spermatozoa (1 × 107 spermatozoa·mL-1) were incubated in BTS with or without varying concentrations of shilajit for 30 min at 37.5℃. The level of intracellular ROS in the sperm was assayed using 1 μM 5-(and-6)-carboxy-2’,7’-dichlorofluorescein diacetate (carboxy-DCFDA; Invitrogen, Eugene, OR, USA). The Ca2+ concentration was measured using 5 nM fluo-3 acetoxymethyl ester (fluo-3/AM; Thermo Fisher Scientific, Seoul, Korea). The fluorescence intensity was measured using a multimode microplate reader (SparkTM 10M, Tekan, Männedorf, Switzerland) with excitation (ex.) at 485 and emission (em.) at 520 nm.
Collection and In vitro maturation (IVM) of pig oocytes
Ovaries were collected from prepubertal gilts at a local slaughterhouse and transported to the laboratory. Cumulus oocyte complexes (COCs) were aspirated from the antral follicles (3 - 6 mm in diameter), washed three times in 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid (HEPES)-buffered Tyrode lactate (TL-HEPES-PVA) medium containing 0.01% (w·v-1) polyvinyl alcohol (PVA), and then washed three times with an oocyte maturation medium (Abeydeera et al., 1998). A total of 50 COCs were transferred to 500 µL of maturation medium covered with mineral oil in a 4-well multi-dish equilibrated at 38.5℃ in an atmosphere containing 5% CO2. The medium used for oocyte maturation was a tissue culture medium (TCM)199 (cat. #50-050-PB; Mediatech, Inc., Manassas, VA, USA) supplemented with 0.1% PVA, 3.05 mM d-glucose, 0.91 mM sodium pyruvate, 0.57 mM cysteine, 0.5 µg·mL-1 luteinizing hormone (LH, L5269, Sigma, St. Louis, MO, USA), 0.5 µg·mL-1 follicle-stimulating hormone (FSH, F2293, Sigma), 10 ng·mL-1 epidermal growth factor (E4127, Sigma), 75 µg·mL-1 penicillin G, and 50 µg·mL-1 streptomycin. After 22 h of culture, the oocytes were cultured in TCM199 without LH and FSH for 22 h at 38.5℃ in an atmosphere containing 5% CO2.
IVF and In vitro culture (IVC) of pig oocytes
After IVM, cumulus cells were removed with 0.1% hyaluronidase in the TL-HEPES-PVA medium (Abeydeera et al., 1998). Thereafter, oocytes were placed into four 100 µL drops of modified Tris-buffered medium (mTBM) covered with mineral oil in a 35-mm polystyrene culture dish. One milliliter of liquid semen preserved in BTS was washed twice with PBS containing 0.1% PVA (PBS-PVA) at 800 × g for 5 min. At the end of the washing procedure, spermatozoa were resuspended in mTBM. After appropriate dilution, 1 μL of the sperm suspension was added to the medium containing oocytes to give a final sperm concentration of 1 × 105 spermatozoa·mL-1. Different concentrations of shilajit (final conc. 0 - 100 µg·mL-1) were added to the fertilization drops at the time of sperm addition during IVF. Oocytes were co-incubated with spermatozoa for 3 hrs at 38.5℃ in an atmosphere containing 5% CO2. After IVF, oocytes were transferred into 500 μL porcine zygote medium (PZM-3; Yoshioka et al., 2002) supplemented with 0.4% bovine serum albumin (BSA, A0281, Sigma) and cultured for an additional 20 hrs (Yi et al., 2021). The IVF study was repeated five times for each treatment regimen involving different concentrations of silajit.
Evaluation of sperm penetration and pronuclear formation
Oocytes/embryos were fixed with 2% formaldehyde for 40 min at room temperature (RT), washed thrice with PBS, permeabilized with PBS-Triton X-100 for 30 min, and stained with 2.5 μg·mL-1 4′,6-diamidino-2-phenylindole (DAPI; DNA staining; Molecular Probes, Eugene, OR, USA) for 40 min. The number of sperm bound to the zona pellucida (ZP) and the fertilization status of the zygotes (unfertilized, fertilized-monospermic, or fertilized-polyspermic) were assessed under a fluorescence microscope (Nikon).
Statistical analysis
Values are expressed as the mean ± standard error of the mean (SEM). Data analyses were carried out using one-way analysis of variance (ANOVA) with the SAS package 9.4 (SAS Institute Inc., Cary, NC, USA) in a completely randomized design. Duncan’s multiple range test was performed to compare the values of individual treatments when the F-value was significant (p< 0.05).
Results and Discussion
As shown in Fig. 1, boar spermatozoa were incubated in the presence of varying concentrations of shilajit. Slightly higher sperm motility and progressive sperm were observed in the sperm incubated with 10 - 50 µg·mL-1 shilajit, but there were no significant differences observed among the treatments (Fig. 1A and B). On the other hand, higher percentages of hyperactive sperm were seen in sperm incubated with 10 and 20 µg·mL-1 shilajit, evidencing that accelerated hyperactivity or enhanced capacitation occurs at these concentrations (p < 0.05; Fig. 1C).
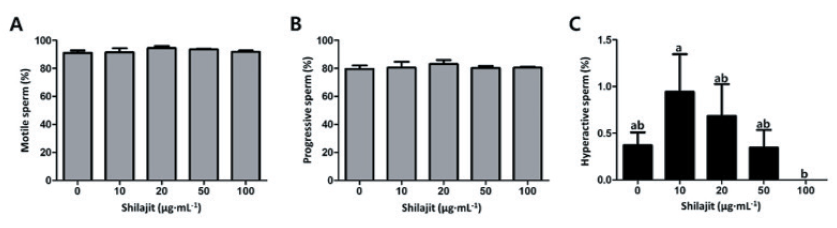
Fig. 1. Comparison of the motility of sperm exposed to varying concentrations of shilajit. After incubation in the presence of shilajit, sperm motility (A), progressive sperm (B), and hyperactive sperm (C) were measured using a computer-assisted sperm analysis system (CASA). Values are expressed as mean ± standard error of the mean (SEM). a, b: Different superscripts in each column indicate significant differences at p < 0.05
Higher intact acrosomes were seen in sperm incubated with 10 - 20 µg·mL-1 shilajit as compared to those in sperm with 100 µg·mL-1 or without shilajit (p < 0.05; Fig. 2A). Higher ATP content was also observed in sperm exposed to 10 µg·mL-1 shilajit (p < 0.05; Fig. 2B). There was a significant decrease in intracellular ROS generation, in sperm incubated with 50 µg·mL-1 shilajit compared to the control sperm without shilajit (p < 0.05; Fig. 3A). Meanwhile, there was an increment in the Ca2+ levels in sperm with shilajit, and significantly higher Ca2+ levels were determined in sperm incubated with 10 or 20 µg·mL-1 shilajit (p < 0.05; Fig. 3B). This suggests a possible role of shilajit in regulating or quenching of ROS generation during sperm incubation while activating cellular changes such as capacitation that allow the sperm to invade the oocyte.
To examine the sperm reaching the oocyte, the number of sperm binding to the zona pellucida (ZP) was counted after 30 min of IVF (Fig. 4A). Higher numbers of sperm were bound to the ZP in the sperm in the presence of shilajit (31.5 - 39.6 in shilajit vs. 27.4 in control). In particular, a significantly higher sperm number was observed in the sperm incubated with 20 µg·mL-1 shilajit (p < 0.05; Fig. 4A). Oocyte and sperm co-incubated in the presence of varying concentrations of shilajit during 3 hrs of IVF, with reduced sperm numbers (1 × 105 sperm·mL-1; Fig. 4B). Increased fertilization rates were observed in the IVF carried out in the presence of shilajit 10 - 100 µg·mL-1 shilajit (65.6 - 87.6%) compared to those in the control IVF carried out without shilajit (61.9%, p <0.05; Fig. 4B). Specifically, higher polyspermic rates were observed in IVF in the presence of 10 - 50 µg·mL-1 shilajit (40.5 - 74.3% vs. 22.8% control, p< 0.05), while monospermic rates (13.3 - 30.1% in 10 - 50 µg·mL-1 shilajit) decreased compared to the control (39.1%, p < 0.05; Fig. 4B). Thus, shilajit enhanced sperm penetration and fertilization. Also, the presence of shilajit effectively reduced the time for sperm penetration and the sperm number required for IVF.
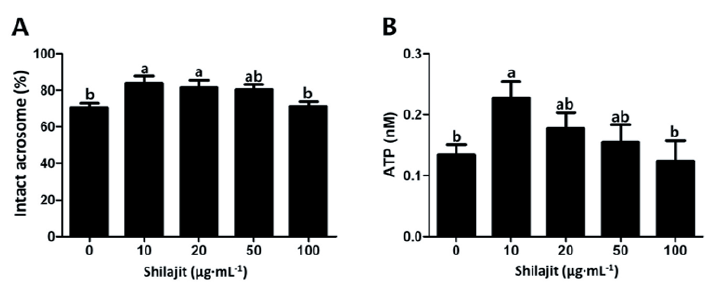
Fig. 2. Acrosomal integrity and adenosine triphosphate (ATP) content of boar spermatozoa exposed to varying concentrations of shilajit. Intact acrosomal spermatozoa are indicated by the fluorescence of peanut agglutinin (PNA [green]) under a fluorescence microscope (A). Higher ATP contents were measured in the sperm incubated with shilajit (B). Values are expressed as mean ± standard error of the mean (SEM). a, b: Different superscripts in each column indicate significant differences at p < 0.05.
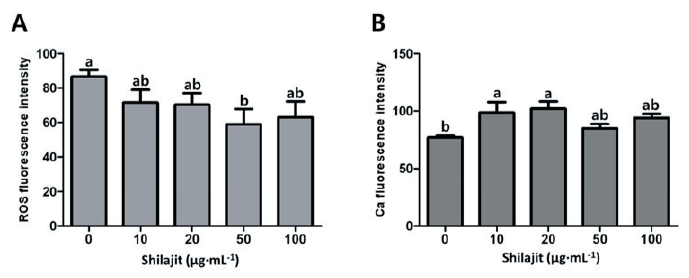
Fig. 3. Intracellular reactive oxygen species (ROS) generation (A) and cytoplasmic Ca2+ level of spermatozoa exposed to varying concentrations of shilajit. Values are expressed as mean ± standard error of the mean (SEM). a, b: Different superscripts in each column indicate significant differences at p < 0.05.
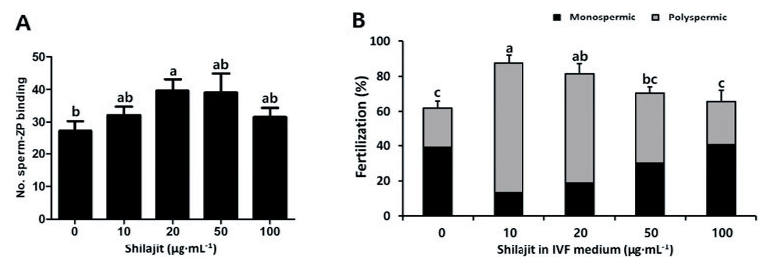
Fig. 4. In vitro fertilization (IVF) in the presence of varying concentrations of shilajit. The sperm attached to the oocyte were counted after 30 min of IVF and staining with 2.5 ㎍·mL-1 4′,6-diamidino-2-phenylindole (DAPI) (A). IVF was performed with reduced sperm number (1 × 105 sperm·mL-1) for 3 hrs. Values are expressed as mean ± standard error of the mean (SEM). ZP, zona pellucida. a - c: Different superscripts in each column indicate significant differences at p < 0.05.
Reproductive inefficiency is one of the most significant reasons for economic loss in the animal industry. Assisted reproductive technology (ART) refers to various techniques including IVF involving the laboratory handling of sperm, oocytes, and embryos that can be used to obtain a large number of viable offspring in sub- or infertile animals or humans (Daly et al., 2020). IVF is a well-established technology with a variety of applications such as the production of embryos for research, treating human infertility, enhancing the productivity of farm animals, and the conservation of endangered mammals (Bavister, 2002). However, conventional IVF is less effective when the semen parameters are below the reference values for concentration, motility, and morphology (Umehara et al., 2018). It has been reported that usually 600 motile sperm per oocyte are required for successful fertilization in conventional IVF protocols for human and mouse oocytes (Magargee et al., 2000). Asthenospermia (the presence of 20% or fewer motile cells in the semen) is a common cause of male infertility, characterized by reduced sperm motility which usually needs to be treated. Oligospermia is a reversible cause of male infertility characterized by low sperm concentration in the semen (less than 10 × 106 sperm·mL-1; Nayak et al., 2019). These conditions contribute to the limited fertilization potential of sperm during IVF, indicating that novel factors are needed to improve IVF media (Umehara et al., 2018).
The present study evaluated the effect of shilajit on the IVF of pig oocytes under reduced sperm concentrations (1 × 105 mL-1) and IVF time (3 hrs). As indicated in Fig. 1C and 4A, the percentage of hyperactive sperm and the number of sperm bound to the ZP increased in the presence of 20 µg·mL-1 shilajit. Shilajit is a rich source of fulvic acid, dibenzo-a-pyrones, and dibenzo-a-pyrone chromoproteins (Agarwal et al., 2007). According to earlier studies, fulvic acids were shown to stimulate respiration in rat liver mitochondria and increased oxidative phosphorylation in concentrations between 40 and 360 mg·L-1, which contributes to increased energy and higher ATP levels (Stohs, 2014). Sperm hyperactivation facilitates sperm penetration into oocytes which can increase the fertilization rate (Fig. 4), suggesting that shilajit can be used as an auxiliary fuel for the spermatozoa. Therefore, positive fertilization rates can be expected if shilajit is used in ART in animals with oligospermia or asthenospermia with low sperm counts and viability.
In general, spermatozoa produce a small amount of ROS during the capacitation process, but a higher amount of free radicals or ROS can damage the plasma membrane and cytoplasm of the spermatozoa (Alvarez and Storey, 1995). It has been shown that high levels of ROS in the semen induce lipid peroxidation, and then negatively affect sperm quality and fertilization (Alvarez and Storey, 1995). Bioactive compounds like free and conjugated di-benzo-alpha-pyrones (DBPs), DBP-chromoproteins (DCPs), and fulvic acids in shilajit can capture ROS and improve sperm health (Biswas et al., 2010). As shown in Fig. 3A, samples treated with shilajit indicated lower fluorescence intensity associated with ROS compared to the sperm in the control group without shilajit. It can be assumed that shilajit acts as a powerful antioxidant in media and improves sperm health and quality. Capacitation is a biological mechanism that acquires spermatozoa to gain the ability to fertilize IVM oocytes, and several chemical substances, such as glycosaminoglycans and heparin, have been shown to induce capacitation (Way and Killian, 2002). As shown in Fig. 3B, Ca2+ levels of sperm incubated with shilajit increased compared to the control without shilajit. An increased Ca2+ level in sperm is associated with capacitation and hyperactivation (Umehara et al., 2018). Thus, the addition of shilajit to IVF media is expected to promote various steps in the sperm fertilization process.
Conclusion
The addition of shilajit during sperm incubation enhanced sperm hyperactivation and reduced the generation of intracellular ROS. Also, the number of sperm binding to the ZP and sperm penetration, thereby increasing the fertilization rate during IVF in the presence of shilajit, suggesting that shilajit can be used as a potential agent during ART to improve the fertilizing capacity of sperm in animals and humans with low sperm number and viability.




