Introduction
Mycotoxins are molecules that are harmful to animal and human health. These are poisonous secondary metabolites of fungi, generally found in grain feeds, which can cause negative impacts on the health and performance of animals (CAST, 2003). Of the 300 to 400 known mycotoxins, deoxynivalenol (DON) and aflatoxin (AF) are the two most common and detrimental mycotoxins to the feed and swine industries (Berthiller et al., 2007; Streit et al., 2012). When pigs consume DON or AF contaminated grain feeds, these mycotoxins can decrease pigs’ growth, cause immune dysfunction or damage to organs (Richard, 2007; Meissonnier et al., 2008; Chaytor et al., 2011). AF and DON-producing fungi grow under different environmental conditions, making it unlikely that these two mycotoxins contaminate grain feeds at the same time. However, there is a great likelihood that grain sources separately contaminated with AF or DON can be mixed during feed processing (CAST, 2003). As a result, pigs can consume a diet contaminated with these two mycotoxins. Together, these mycotoxins may act synergistically to affect animals (Kouadio et al., 2005; Miazzo et al., 2005). Because of its ability to adsorb most poisonous compounds, charcoal has been used in medicine for the last 100 years (Chyka et al., 2005). In the mid-1970s, activated charcoal was accepted as an antidote in adsorption and elimination of a variety of medications (Davis, 1991). Furthermore, charcoal has the ability to adsorb toxic gases (Seo and Lee, 2010). The adsorptive power of charcoal can be increased considerably when treated with various substances at temperatures ranging from 500 to 900°C. The final product of this process is called ‘activated charcoal’ (Osol, 1975). Practical methods to detoxify mycotoxin contaminated grain on a large scale and in a cost-effective manner are not currently available. A current approach is to add non-nutritive feed additives, such as sorptive materials, to the diet in order to reduce the absorption of mycotoxins in the gastrointestinal tract. Organic medicinal charcoal added to grain feeds may have the capability to reduce the impact of mycotoxins through its binding properties (Jones et al., 1994). Moreover, all charcoal additives containing pyroligneous acid and coconut tree charcoal are known to enhance fatty acid composition, improve fattening performance, and have fecal deodorizing effects (Ahn et al., 2005). The efficacy of these as additives depends on their concentration in the feed. Hence, this present study was done to investigate the possibility of utilizing charcoal in the pig feed additive industry.
Materials and Methods
Experimental design, animals and diets
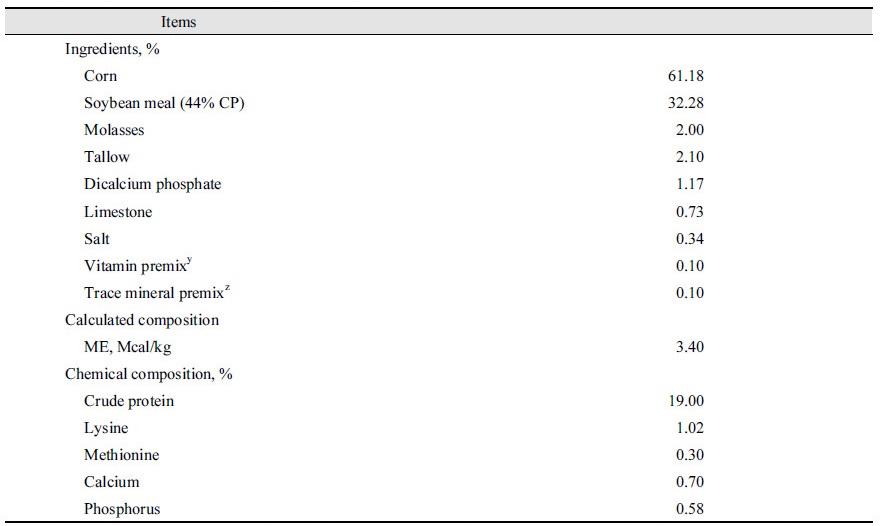
A 10-day study with a total of 20 crossbred pigs [(Yorkshire × Landrace) × Duroc] with an initial body weight (BW) of 81.0 ± 3.3 kg were randomly assigned to 4 dietary treatments, with 8 and 20 replications, for in vivo and in vitro experiments, respectively.
Each pen was equipped with a 1-sided, stainless-steel self-feeder and a nipple drinker that allowed pigs ad libitum access to feed and water. Treatments for in vitro and in vivo experiments included: control basal diet (CON ), 0.25% Organic Medicinal Charcoal-supplemented basal diet (OMC), 0.50% Pyroligneous Charcoal-supplemented basal diet (PC), and 0.50% Coconut tree Charcoal-supplemented basal diet (CC) (Table 1).
Sampling and measurements
Swine odor in fecal matter
Fresh feces samples (300 g) were collected by anal massage method into sealing plastic boxes (polyvinyl; 2,600 mL) for analyzing odor substances excreted in the minutes the same time diet after feeding for 10 days. The following 4 gases were measured using a GV-100 gas sampling pump and detector tube system (Gastec, Japan): NH3, H2S, Total mercaptans, and Acetic acid.
Fecal microflora in vivo
Fresh feces samples (minimum 10 g) were collected daily by anus massage method, after feeding, for 10 days. Samples were stored at -20°C until analysis. Before analysis, they were thawed, suspended in sterilized physiological saline, and then homogenized. Finally, the samples were serially diluted in steps resulting in 103 to 107 -fold dilutions for measuring the number of viable cells with eyes.
Aflatoxin adsorption
The aflatoxin adsorption capacity of charcoal was measured using an ELISA test kit (RIDASCREEN, Dermstadt, Germany).
In vitro nutrient digestibility
This study was conducted following the method described by Cho and Kim (2011).
Step 1: Approximately 0.5 g of feed was weighed within an accuracy of ± 0.1 mg into 100 mL conical flasks with a blank included in each series. Each diet had 20 replicates because 0.5 g of feed was not enough for analysis. A small magnetic rod and 25 mL of phosphate buffer (0.1 M, pH 6.0) were added to each flask, the sample and buffer were then mixed carefully by gentle magnetic stirring. A volume of 10 mL of 0.2 M HCl was added to the slurry, and then pH was adjusted to pH 2 using a 1 M HCl or 1 M NaOH solution. A volume of 1 mL of freshly prepared pepsin solution containing 10 mg pepsin (porcine, 2000 FIP U/g, Merck art no. 7190) was then added to the mixture. In order to prevent bacterial growth, 0.1 mL of a chloramphenicol solution (0.5 g chloramphenicol (ICN no. 190321) per 100 mL ethanol) was also added to the mixture. The flasks were then closed with a rubber stopper and incubated in a heating chamber at 40°C for 75 min with constant magnetic stirring.
Step 2: After incubation, 10 mL of a phosphate buffer (0.2 M, pH 6.8) plus 5 mL of a 0.6 M NaOH solution were added. The slurry was adjusted to a pH of 6.8 with a 1 M HCl or 1 M NaOH solution, and then mixed with 1 mL freshly prepared pancreatin solution containing 50 mg pancreatin (porcine, grade IV, Sigma no p 1750). After closing flasks with a rubber stopper, the sample was incubated under constant magnetic stirring in a heating chamber at 40°C for three hours and thirty minutes. A minimum of 0.5 g diatomaceous earth (celite 545, Tecator, Sweden) was added to glass filter crucibles and rinsed. Then, samples were dried at 100 °C for at least 4 hours and crucibles were weighed after cooling in a desiccator. The undigested residues were then collected in a filtration unit (Fibertee System M, Tecator, Sweden) using the dried and pre-weighed glass filter crucibles (d: 3 cm; pore size: 40 - 90 pm), containing diatomaceous earth as a filter aid, described above. All material was then transferred with 1% sulphosalicylic acid to the crucible. After consecutive washings with 10 mL of ethanol and acetone, the crucible was suctioned (with a water pump) to be as dry as possible. The undigested residues were then dried at 103°C overnight. The crucible was placed in an ashing oven and its content was ashed at 550°C for about 4 hours. After ashing, the crucibles were cooled in a desiccator and subsequently weighed.
Statistical Analysis
All data were subjected to statistical analyses as a randomized complete block design using the GLM procedure of the SAS software (SAS Institute, 1996). Differences among treatment means were determined using Tukey’s Multiple range test.
Results and Discussion
Swine odor in fecal matter
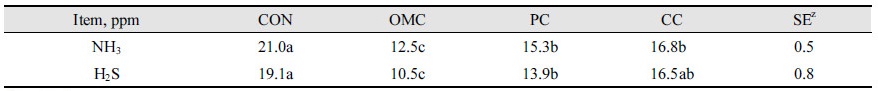
The results of the 10 day feeding trial are represented in Table 2. The effect of a diet supplemented in OMC, PC, and CC on fecal gas emissions of finishing pigs was analyzed. Fecal ammonia and H2S emission of OMC were significantly lower than those of CON, PC, and CC (p < 0.05). Ammonia emissions of feces were 21.0, 12.5, 15.3, and 16.8 in CON, OMC, PC, and CC, respectively. H2S emissions of feces were 19.1, 10.5, 13.9, and 16.5 in CON, OMC, PC, and CC, respectively.
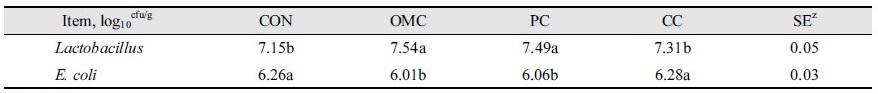
The results of the Lactobacillus counts in feces are presented in Table 3. The number of Lactobacillus in feces for OMC and CC were significantly higher than for CON and PC (p < 0.05). The number of fecal E. coli for OMC and CC were lower than for CON and PC (p < 0.05). The number of Lactobacillus in feces were 7.15, 7.54, 7.49, and 7.31 in CON, OMC, PC, and CC, respectively. The number of E. coli in feces were 6.26, 6.01, 6.06, and 6.28 in CON, OMC, PC, and CC, respectively.
Organic medicinal charcoal decreased fecal ammonia and H2S emissions of finishing pigs in the present experiment. Generally, wood charcoal has the best potential to provide a way to negative effects of gases. It also offers an economical way to eliminate noxious substances (Kutlu et al., 2001). Activated charcoal has been shown to adsorb a wide range of compounds although adsorptive capacity of activated charcoal depends on the pore size of activated charcoal (Struhsaker et al., 1997). Adsorption therapy with activated charcoal as a non-digestible carrier is one of the most important methods of preventing the negative effects of ingested toxicants or noxious substances formed in the gastrointestinal tract (McKenzie, 1991; Jindal et al., 1994). In the present study, similarly to several other studies, fecal ammonia and H2S emissions decreased with the addition organic medicinal charcoal.
Fecal microflora
The results of the Lactobacillus counts in feces are presented in Table 3. The number of Lactobacillus in feces for OMC and CC were significantly higher than for CON and PC (p < 0.05). The number of fecal E. coli for OMC and CC were lower than for CON and PC (p < 0.05). The number of Lactobacillus in feces were 7.15, 7.54, 7.49, and 7.31 in CON, OMC, PC, and CC, respectively. The number of E. coli in feces were 6.26, 6.01, 6.06, and 6.28 in CON, OMC, PC, and CC, respectively.
It was reported that beneficial bacteria, such as Lactobacillus and Bifidobacterium, can enhance the metabolism of host birds and improve gut efficiency by increasing nutrient absorption (Gabriel et al., 2006) and accelerating gut development (Furuse et al., 1991). Whereas, harmful bacteria damage the villi and microvilli of intestinal mucosa, inhibit the secretion of digestive enzymes (Xu et al., 2003), and produce gastrointestinal diseases (Fairbrother and Gyles, 2006; Song et al., 2015). As a result, beneficial bacteria may reduce intestinal colonization and slow infectious processes, thereby decreasing the inflammatory process in the intestinal mucosa, which improves villi height and secretory functions, digestion, and absorption of nutrients (Iji and Tivey, 1998). Poage et al (2000), reported that bamboo charcoal increased the beneficial bacteria (Lactobacillus spp.) and decreased the harmful bacteria (E. coli and Salmonella spp.) in fattening pigs. Activated charcoal decreased toxins in ruminants. Bacterial toxin (Buck and Bratich, 1986) and E. coli (Naka et al., 2001). Buck and Bratic (1986) reported the effectiveness of activated charcoal to reduce gastrointestinal tract infections caused by bacterial toxins in human. Miner (1975) summarized several studies showing that attempts to reduce odors by livestock fed various microbial organisms or physical additives were not successful.
In the present study, an abondance of fecal Lactobacillus were found to be present in feces of pigs fed organic medicinal charcoal and coconut tree charcoal, and their counts were higher than those obtained from basal diet feces. Also, the number of fecal E. coli of feces from organic medicinal charcoal treatment and coconut tree charcoal treatment were lower than those of feces from the basal diet.
Aflatoxin adsorption
The results of aflatoxin absorption capacity by different types of charcoals are represented in Fig. 1. In vitro, the aflatoxin absorption capacity was 100, 10, and 20% in organic medicinal charcoal, pyroligneous charcoal, and coconut tree charcoal, respectively.
One of most effective, nontoxic, nonspecific binding sorbent is activated charcoal. It has a high surface-to-mass ratio (500 - 3500 m2/g) and contains binding agents formed by pyrolysis of various organic compounds. It adsorbs zearalenone, deoxynivalenol, and nivalenol in vitro in a gastrointestinal model and aflatoxins, ochratoxin A, and fumonisins in vivo (Pettersson, 2004; Kolosova and Stroka, 2012).
In vitro, different active charcoals adsorb aflatoxins at different pH (Huwig et al., 2001). In vivo, the conversion of aflatoxin M1 decreased in Friesian cows by 41 - 71% when some granulated activated carbon was included in the diet at 2% of aflatoxin (Richard et al., 2003). Chicken body weight and feed intake increased when activated charcoal was added to aflatoxin-contaminated diets (Dalvi and Ademoyero, 1984).
In vitro nutrient digestibility
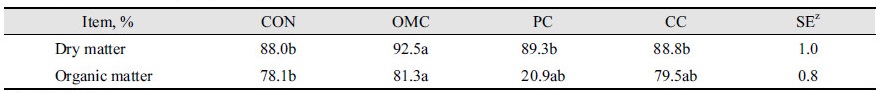
The result of supplementation of OMC, PC, and CC on in vitro digestibility was represented in Table 4. The digestibility of dry matter in OMC was significantly higher than CON, PC, and CC in vitro (p < 0.05). The digestibility of organic matter was observed as 78.1, 81.3, 80.9, and 79.5% in CON, OMC, PL, and CC, respectively. OMC treatment results were significantly different than those of CON (p < 0.05).
The extreme inertness of charcoal makes it an unlikely source of minerals when passed through the mammalian digestive system (Cooney, 1995). Activated charcoal controls the lactic acid concentration by maintaining pH levels and microflora population in non-ruminant and ruminant. It also combines with phenol in gastrointestinal tracks, which prevent interference of hydrosoluble tannins with enzymatic function and protein digestion (Murdiati et al., 1991). Absorption therapy with activated charcoal as a non-digestible carrier is one important method for inactivating ingested toxicants or noxious substances from the gastrointestinal tract (Zhao et al., 2008). There is evidence that suggests that microwave-activated bamboo charcoal particles improve immune performance, enhance absorption in the small intestine, decrease radical production, and maintain/ameliorate cellular oxidative phosphorylation levels (Wang et al., 2012). Bamboo charcoal has also been studied as an animal feed additive. For example, bamboo charcoal used as an antibiotic alternative in the diet of fattening pigs, leads to better growth performance, immune responses, and fecal micro-flora populations (Chu et al., 2013). There is evidence showing that the incorporation of bamboo charcoal into the diet of piglets activates intestinal function, both at the level of intestinal villi, as well as at the cellular level, resulting in a trend for improved feeding efficiency (Mekbungwan et al., 2004). Finally, supplementing the diet with 0.5% bamboo charcoal was found to achieve maximum growth performance and to decrease the ammonia nitrogen excretion of flounder (Moe, 2010).
Some researchers studied charcoal and activated charcoal as animal feed additives. Charcoal affected growth performance and carcass traits in fattening pigs (Hwang, 1995), and was used as feed additive for production of high-quality meat (Kim, 1990). Activated charcoal also affected microbe reproduction in sheep (Knutson et al., 2006) and meat quality and storage characteristics of pork (Lee et al., 2011). In this study, the digestibility of organic medicinal charcoal on dry matter was higher than that of basal diet in vitro.