Introduction
Weaning is a nutritionally, physiologically, and environmentally critical period in pig production. To reduce digestive disorders and diarrhea, previous studies have investigated effects of changes in ingredient compositions and nutrient levels including protein and carbohydrates in weaning pigs (Montagne et al., 2004; De Lange et al., 2010; Mun et al., 2017). Even though weaning pigs require high amounts of dietary protein, the negative effects of high protein content, including diarrhea and reductions in growth rates, can be observed during the first two weeks after weaning
(Madec et al., 2000; Jang et al., 2016). A high amount of protein may encourage the proliferation of pathogenic bacteria in the gastrointestinal tract (Ball and Aherne, 1987). However, the increase in protein level (obtained by increasing in wheat or corn gluten meal concentrations) in the weaning pigs’ diet leads to increased daily gain and body weight (Bikker et al., 2006). Reducing the amount of protein in the diet of weaning pigs by more than 2% of the standard level had a negative impact on pigs’ growth (Nyachoti et al., 2006). This decreased growth performance was specifically investigated during the first one or two weeks after weaning. Wellock et al. (2009) suggested that the growth retardation was related not only to protein quantity, but also to protein quality. Wellock et al. (2009) found that the low protein quality in a diet with raw cereal and plant protein had a negative effect on the growth of weaning pigs over the short term.
Previous research attempted to balance intestinal microbes by reducing proteins, coupled with amino acid supplementation (Le Bellego et al., 2002; Nyachoti et al., 2006; Le Wellock et al., 2007; Heo et al., 2009). On the other hand, dietary fiber has been investigated for its capacity to reduce the chance of metabolic disorders and to improve the microbial balance in young pigs (Mateos et al., 2006; Wellock et al., 2008; Molist et al., 2009; Halas et al., 2010). The beneficial effects of dietary fiber include an improved gut ecosystem and the nitrogen repartitioning effect. Therefore, the objective of this study was to investigate the effects of different levels of dietary fiber and protein in diets on the growth performance and fecal characteristics of weaning pigs.
Materials and Methods
The Animal Care and Use Committee of Dankook University approved all experimental protocols used in the current study.
Experimental design, animals and diets
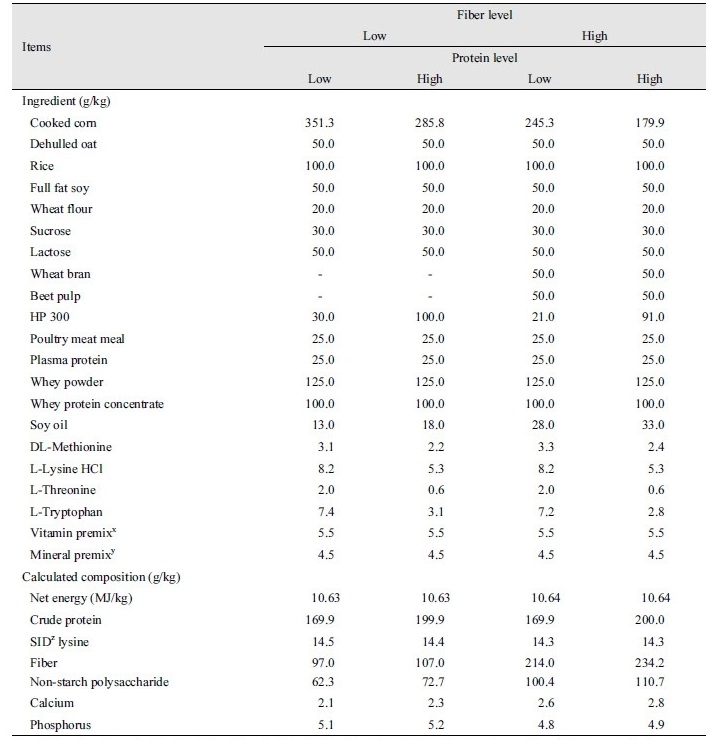
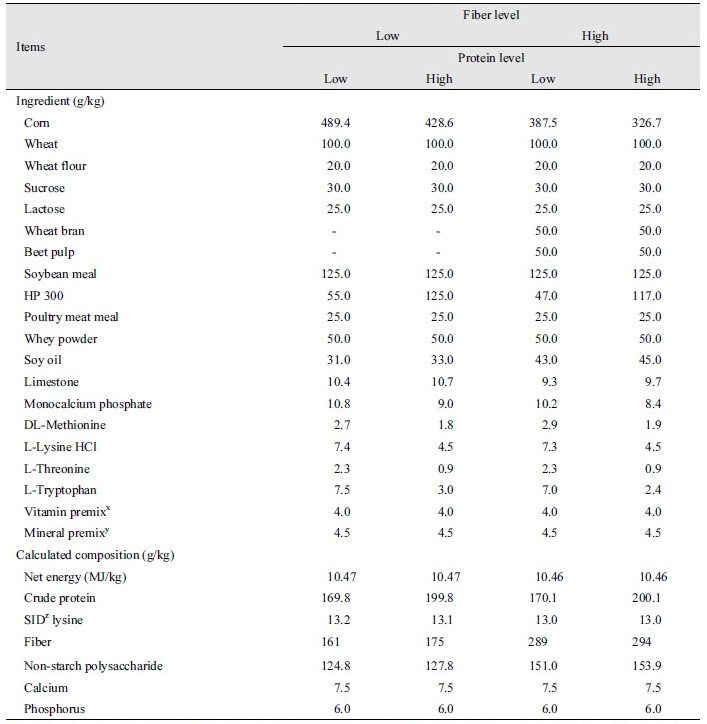
A total of 96 barrows ([Landrace × Yorkshire] × Duroc) with an average initial body weight of 7.41 ± 0.71 kg were selected for a 5-week trial. Pigs were allotted to dietary treatments based on their body weight and sex in a 2 × 2 factorial experiment, with the respective factors being fiber (low vs. high; 100 and 200 g/kg, respectively, during days 0 to 14; 175 and 300 g/kg, respectively during days 14 to 35, respectively) and protein (low vs. high; 170 and 200 g/kg respectively). Each treatment had 6 replicate pens (1.0 × 1.2 m2) consisting of 4 pigs (2 gilts and 2 barrows) per pen. Low protein diets included a balanced level of lysine combined with synthetic amino acids. Wheat bran and beet pulp were used as fiber sources in the experimental diets. All experimental diets were fed in mash form. All of the diets were formulated to contain approximately 10.63 MJ/kg net energy and 14.3 g/kg standard ileal digestible basis (SID) lysine for phase I (days 0 to 14), and 10.46 NE MJ/kg and 13.0 g/kg SID lysine for phase II (days 14 to 35) (Tables 1 and 2). Diets were formulated to meet or exceed the nutrient requirements recommended by NRC (2012). All of the pigs were housed in an environmentally controlled facility with slatted plastic flooring and a mechanical ventilation system. Each pen was equipped with a one-sided self-feeder and a nipple waterer to allow the pig ad libitum access to feed and water throughout the experimental period.
Sampling and measurements
Individual pig weight was measured at the beginning, day 14, and day 35 of the experimental period, and feed consumption was recorded on a pen basis to calculate average daily gain (ADG), average daily feed intake (ADFI), and gain : feed ratio.
Subjective fecal scores were monitored daily from days 0 to 35 by the same trained person using a scale ranging from 1 to 5, insert a space behind where 1 = hard and dry pellet but small mass, 2 = hard and formed stool, 3 = soft and formed stool but moist, 4 = soft and unformed stool, and 5 = watery and liquid stool.
For fecal microbial shedding, fecal samples were collected via massaging the rectum from 2 pigs randomly selected from each pen (1 gilt and 1 barrow), pooled and placed on ice for transportation to the laboratory, where analysis was immediately carried out. The composite fecal sample (1 g) from each pen was diluted with 9 mL of 1% peptone broth (Becton, Dickinson and Company, NJ, USA) and homogenized. Viable counts of bacteria in the fecal samples were then conducted by plating serial 10-fold dilutions (in 1% peptone solution) onto MacConkey agar plates (Difco Laboratories, Detroit, MI, USA) and Lactobacilli medium III agar plates (Medium 638, DSMZ, Braunschweig, Germany) to isolate the Escherichia coli and Lactobacilli, respectively. The MacConkey agar plates were then incubated for 24 h at 37℃, and the Lactobacilli medium III agar plates were incubated for 48 h at 39℃ under anaerobic conditions. The E. coli and Lactobacilli colonies were counted immediately after removal from the incubator.
Statistical analyses
Before conducting statistical analysis of the microbial counts, the value was transformed logarithmically. All data were analyzed as a completely randomized 2 × 2 factorial arrangement by using the GLM procedure of SAS (SAS Inst. Inc., 2009). The pen was considered the experimental unit. The final model included the main effects of protein and fiber levels as well as the interaction between protein and fiber levels. The data were reported as means with their standard errors. Probability values less than 0.05 were considered significant.
Results
Growth performance
The effect of different levels of dietary fiber and protein on the growth of weaning pigs is shown in Table 3. No significant differences were observed between the initial and final body weights, ADG during phase II and overall period, ADF during phase I and overall period, and gain:feed ratio through the experiment. However, on day 14, pigs fed with high protein or high fiber diets were heavier (p < 0.05) than those fed with low protein or low fiber diets. During days 0 to 14, the ADG of pigs fed higher protein diets was higher than those fed low protein diets (p < 0.05). Interactive effects of fiber and protein were only found on ADFI during days 14 to 35 (p < 0.05).
|
Table 3. Effects of levels of fiber and protein on growth performance in weaning pigs. 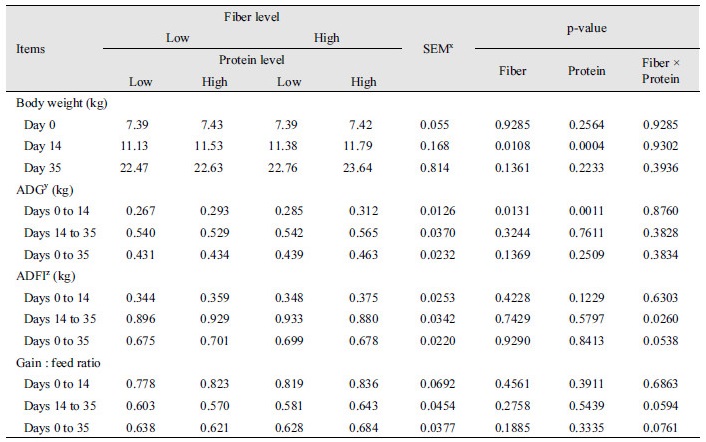
|
|
|
xSEM: standard error of the mean. yADG: average daily gain. zADFI: average daily feed intake. |
|
Fecal scores and fecal microbial shedding
The effect of dietary fiber and protein levels on fecal scores and fecal microbial shedding are shown in Tables 4 and 5, respectively. Although the pigs were fed different levels of fiber and protein, there was no diet-related difference in fecal scores in this study. On day 14, pigs fed a high fiber diet had lower counts of E. coli (p < 0.05) than those fed low fiber diets. However, the fiber level did not affect Lactobacilli counts. On day 35, more E. coli was found in the feces of pigs fed with high protein or low fiber diets (p < 0.001). Interactive effects of fiber and protein levels were not found in microbial counts on both days 14 and 35.
Discussion
Livestock production can make good use of resources and contribute high quality meat to the human diet (Mohana Devi et al., 2014). The effect of different levels of fiber and protein on the growth of weaning pigs was studied in the present study. In our study, 200 g/kg protein in the diet was considered as the high protein diet and 170 g/kg protein was the low protein diet. No significant differences were observed in final body weight, ADG (days 14 to 35 and 0 to 35), ADFI, and feed efficiency. However, on day 14, pigs fed with high protein or high fiber diets were heavier than those fed with low protein diet or low fiber diets. During days 0 to 14, the ADG of pigs fed higher protein diets was higher than that of those fed low protein diets. The interactive effects of fiber and protein were only found in ADFI during days 14 to 35. A previous study showed that reduction of crude protein content from 187 g/kg to 171 g/kg in the diet did not affect growth performance if crystalline isoleucine and valine were added into the low protein diet (Norgarrd et al., 2009). In several studies, increased dietary fiber was associated with decreased ADG in young pigs. Hedemann et al. (2006) reported a decrease in the ADG and ADFI of weaning pigs fed diets that had dietary fiber contents increased from 73 to 104 or 145 g/kg. In contrast, other studies indicated that no adverse effects on growth performance were observed when pigs were fed different dietary fiber levels. Molist et al. (2009) reported that there was no significant difference in ADG when pigs with a body weight of 7.4 kg were fed a cereal-based diet containing 8% wheat bran, 6% sugar beet pulp or 4% wheat bran, and 3% sugar beet pulp. Weber et al. (2008) tested different fiber sources such as corn distillers, soybean hulls and citrus pulp in weaning pigs. They found that feeding 7.5% of each fiber source did not affect growth performance. Mateos et al. (2006) found that increments in dietary fiber in the diet improved the apparent total tract digestibility coefficients of organic matter, crude protein, and energy.
Although pigs were fed with different levels of fiber and protein, there was no diet-related difference in fecal score. On day 14, pigs fed high fiber had lower counts of E. coli than those fed with low fiber diets. However, fiber level did not affect the Lactobacilli counts. There was no significant difference in the number of Lactobacilli and E. coli for the different protein treatments. On day 35, more E. coli were found in the feces of pigs fed high protein or low fiber diets compared to that of pigs fed low protein or high fiber diets. Lactobacilli counts in feces were not affected by low protein or high fiber treatments. No interactive effects of fiber and protein levels were found on microbial counts in feces on days 14 and 35. Protein fermentation in the digestive tract is considered as the driver for the proliferation of pathogenic bacteria such as E. coli (Ball and Aherne, 1987; Wellock et al., 2009). Dietary fiber selectively supports the growth of Lactobacilli (Konstantinov et al., 2004). Thus, we expected that when piglets were fed a low protein or high fiber diet, the numbers of Lactobacilli and E. coli would be positively affected, thereby improving growth performance. In the present work, we found that increasing fiber contents in the diet of post-weaning piglets improved ADG during days 0 to 14 and this result was consistent with the observations from Molist et al. (2009), Halas et al. (2010), and Weber and Kerr (2011). It may be that more fiber in the diet improved the gut beneficial microflora, which provides additional energy to the host by the fermentation of fiber. Though fiber is reported to have negative effects on small intestinal and total tract absorption of dietary lipids and energy (Wilfart et al., 2007), it can be utilized by most Bifidobacteria, Bacteroides and some Streptococci, Lactobacilli and Enterobacteria, but not E. coli (Hidaka et al., 1986). Increased fermentation of beneficial microbes significantly improves the growth performance of young piglets (Hermes et al., 2009). However, it was found that the degree of fermentation and its relationship with the growth of piglets depend on the amount and sources of dietary fiber (Williams et al., 2001).