Introduction
Keeping pigs healthy is a considerable challenge. The swine industry has widely used antibiotics as antimicrobials to prevent or control diseases, to suppress or inhibit the growth of certain microorganisms, or to promote growth via their inhibition of the normal microbiota, which subsequently improves growth rate, efficiency, and reproductive performance and reduces mortality and morbidity (Cromwell, 2002; Gaskins et al., 2002). However, safety issues associated to the use of antibiotics in livestock have been raised, such as their potential threat to the consumer’s health (human) and the potential development of antibiotic-resistant enteric bacteria, which pose a potential animal and human health risk (Cromwell, 2002; Hardy, 2002; Pluske et al., 2002).
These issues have made the use of antibiotics for livestock animals more restricted and may bring about a complete ban in Korea as well as in the European Union. Therefore, the swine industry has considered various alternatives to improve pig health without the potential safety issues that come with antibiotic use.
It is imperative that the swine industry prepares for such restrictions in case they occur. Recently, the swine industry increasingly considers the use of dietary factors like feed ingredients, feed additives, feed formulation practices, or feeding methods to improve pig health and performance (Pluske et al., 2002; Pettigrew, 2006; Stein and Kil, 2006). There are now remarkably rich supplies of products and practices available to the swine industry and they are being proposed to improve pig health as well as productive performance.
Spray-dried plasma (SDP) may be a candidate among the alternatives to antibiotics, providing bioavailable nutrients and several physiological components to modulate intestinal microbiota and immunity. In this review, the value of spray-dried plasma supplement in swine diets is described.
Definition of spray-dried plasma
SDP is made from blood collected at slaughter plants. An anticoagulant, sodium citrate, is added to the blood, and the plasma is separated by centrifugation and, subsequently, spray-dried (van Dijk et al., 2001; Pettigrew et al., 2006).
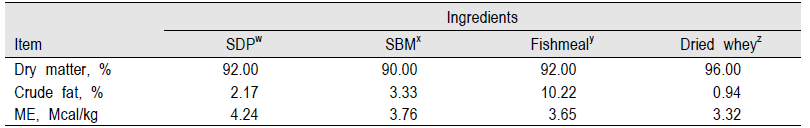
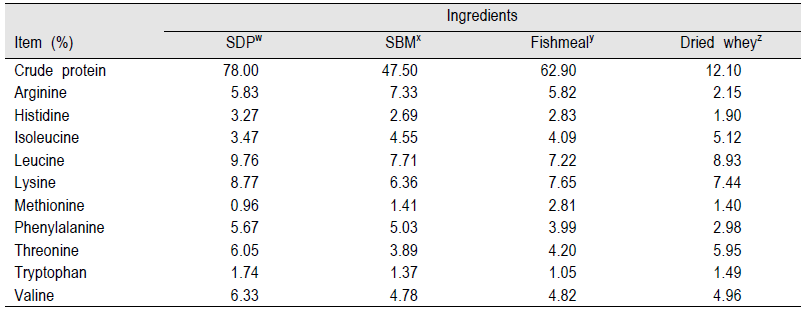
SDP is an excellent nutrient source for nursery pigs because of an excellent balance of essential amino acids with high digestibility (over 70%). It contains highly metabolizable energy (ME) (about 4 Mcal/kg) compared with soybean meal, which is one of the main protein and energy sources in pig diets (Table 1, 2; NRC, 1998). SDP is also a complex mixture of many physiological components including immunoglobulins, glycoproteins, albumin, growth factors, peptides, and other physiologically active components (Coffey and Cromwell, 2001; Markowska-Daniel and Pejsak, 2006; Moreto and Perez-Bosque, 2009). Based on established benefits, the swine industry has used SDP commercially to improve growth rate, feed intake, and feed efficiency and to reduce mortality and morbidity of early-weaned pigs.
General proposed mechanisms of action of spray-dried plasma
SDP has several potential effects when included in pig diets. Firstly, SDP improves growth performance by increasing feed intake, average daily gain, and gain:feed ratio (efficiency) (Coffey and Cromwell, 2001; van Dijk et al., 2001; Pettigrew, 2006) because of immune-competence or high palatability (Ermer et al., 1994), but the mechanism of action is not clear. Secondly, SDP improves pig health by protective effects against diseases including post-weaning diarrhea (Coffey and Cromwell, 2001; van Dijk et al., 2001; Pettigrew et al., 2006) as SDP provides physiological components such as immunoglobulins, glycoproteins, or others that adhere to some pathogens and prevent the colonization of enterocytes by the pathogens. Thirdly, SDP improves the intestinal barrier function (Perez-Bosque et al., 2006; Moreto and Perez-Bosque, 2009) by modulation of intestinal immune systems or by some physiologically active components in SDP, but the mechanism of action is not clear.
Effects of spray-dried plasma on pig performance and health
SDP has been used as one of the main protein sources in nursery pig diets because of its provision of bioavailable nutrients as well as physiologically active components in the SDP. It has been reported in previous review papers that SDP improves the growth rate of weaned pigs by increasing feed intake, through immune-competence or high palatability, by about 25% (Coffey and Cromwell, 2001), 27% (van Dijk et al., 2001), and 23% (Pettigrew, 2006), compared with control diets. In addition, those benefits are more pronounced in a conventional or non-sanitary environment (Coffey and Cromwell, 1995; Zhao et al., 2008). Based on the evidence, it is clear that SDP is beneficial for nursery pigs against limited growth rates and disease susceptibility during weaning transition (Pettigrew et al., 2006).
SDP can also improve intestinal morphology (Owusu-Asiedu et al., 2003a,b; Carlson et al., 2005; Bhandari et al., 2008) as reported in studies in which increased villous height, decreased crypt depth, or increased villous height:crypt depth ratios were observed. However, these results were not consistently observed in other studies (Jiang et al., 2000; Touchette et al., 2002; Nofrarias et al., 2006). The improvement of intestinal morphology can enhance nutrient absorption, resulting in superior growth performance.
One of the main potential effects of SDP is an antigen-antibody interaction or anti-bacterial effect in the digestive tract. With pathogenic E. coli challenges generally causing diarrhea problems and further mortality or morbidity in weaning pigs, SDP improves growth performance (Nollet et al., 1999; van Dijk et al. 2002; Bosi et al., 2004), reduces diarrhea scores (Niewold et al., 2007; Bhandari et al., 2008) and reduces pathogenic E. coli counts in feces (Nollet et al., 1999; Owusu-Asiedu et al., 2003a,b; Niewold et al., 2007). It also reduces mortality (Nollet et al., 1999; Owusu-Asiedu et al., 2003a,b; Bhandari et al., 2008) by the inhibition of pathogen-binding to the intestinal epithelial cells through the action of immunoglobulins or glycoproteins present in SDP (Nollet et al., 1999; Pettigrew et al., 2006).
SDP modulates inflammatory responses. Some reports show SDP reduces intestinal wall thickness, villous width, and lamina propria surface area (Jiang et al., 2000; Carlson et al., 2005; Nofrarias et al., 2006), by possibly suppressing inflammation. Nofrarias et al. (2006) also showed SDP modulates the intestinal immune system by reducing immune cell subsets (monocytes, macrophages, B lymphocytes, γδ+ T cells, etc.) in blood and ileal Peyer’s patches, but Zhao et al. (2008) did not observe SDP effects on pro- and anti-inflammatory cytokine mRNA expressions in the small intestine of nursery pigs after weaning.
With challenges, several reports show that SDP modulates the intestinal immune system by reducing the expression of pro-inflammatory cytokine mRNA against a pathogenic E. coli (Bosi et al., 2004) and lipopolysaccharide (LPS) challenges in tissue (Touchette et al., 2002). Moreover, SDP reduces acute phase proteins and TNF-α mRNA expression (Frank et al., 2003) in environmental stress (low temperature), perhaps by inhibiting pathogenic microbial growth or colonization in the intestine, and consequently improving mucosal integrity. Therefore, energy can be diverted from the activation of the immune system to growth (Touchette et al., 2002; Nofrarias et al., 2006).
On the other hand, SDP makes pigs more susceptible to overstimulation of serum pro-inflammatory cytokines against LPS challenge (Touchette et al., 2002) and cold stress with LPS challenge (Frank et al., 2003), resulting in major damage to the mucosa of the gastrointestinal tract, and to the activation of stress responses in serum in the hypothalamic-pituitary-adrenal axis (Carroll et al., 2002) against LPS challenge.
The intestinal barrier consists of secreted mucus, immunologic factors, as well as enterocyte membranes and tight junctions between enterocytes in the intestinal epithelium (Lambert, 2009). It is a selective barrier to allow the uptake of nutrients and to prevent (or not to allow) biological and chemical agents (e.g., food antigens, endotoxins, hydrolytic enzymes, intestinal microbes, etc.) across the epithelium (Lambert, 2009; Moreto and Perez-Bosque, 2009). The integrity of this barrier can be made dysfunctional by physiological, pathological, psychological, or pharmacological stress (Lambert, 2009). It leads to increased intestinal permeability to biological and chemical agents by diminishing the interlocking proteins related to the tight junctions, which causes local and/or systemic inflammatory reactions (Moreto and Perez-Bosque, 2009). Inflammatory reactions induce the production of pro-inflammatory cytokines followed by inflammatory cell recruitment. The inflammatory cells release reactive oxygen species to eliminate the pathogens, but the reactive oxygen species also cause tissue damages (Perez-Bosque et al., 2006; Moreto and Perez-Bosque, 2009). Thus, it is beneficial for the maintenance of intestinal barrier integrity during stresses if the diminished tight junction proteins are prevented and/or the pro-inflammatory cytokines are suppressed during inflammation.
Perez-Bosque et al. (2006) showed that SDP improves intestinal barrier functions during intestinal inflammation, using rats challenged with Staphylococcus aureus enterotoxin B. SDP prevents reductions of proteins such as ZO-1 (tight junction protein) and β-catenin (adherent junction protein) and reduces intestinal permeability, which is measured by the passage of high molecular weight probes (Lambert, 2009; Moreto and Perez-Bosque, 2009) across the intestinal barrier.
Studies using rats challenged with Staphylococcus aureus enterotoxin B showed that SDP also reduces pro-inflammatory cytokines (IFN-γ and IL-6) in the intestinal mucosa and Peyer’s patches (Moreto et al., 2008) and T-helper lymphocytes and γδ-T lymphocytes in the gut-associated lymphocyte tissues (Peyer’s patches, lamina propria, and intraepithelial compartments) (Perez-Bosque et al., 2004, 2008). However, SDP increases anti-inflammatory cytokine (IL-10) in the intestinal mucosa (Moreto et al., 2008).
Conclusion
Spray-dried plasma is a blood product that provides bioavailable nutrients as an excellent protein source with balanced and highly digestible amino acids. Furthermore, it supplies physiologically active components such as immunoglobulins, glycoproteins, growth factors, peptides, etc. Several beneficial physiological activities are based on those components of spray-dried plasma, such as immune competence (antibacterial activity), modulation of microbiota and/or immune system, and the maintenance of intestinal barrier integrity and function. Those beneficial effects can contribute to the improvement of pig performance and health by the regulation of microbiota in the digestive tract and/or that of the immune system.